You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
Shiseido Hair Loss Clinical Trial To Begin
- Thread starter Swoop
- Start date
- Reaction score
- 119
That is great! The sooner it is available in Europe and other parts of the world, the better (if it works off course).
Even if it is in Japan first, it might not be possible for all people to travel there to get the treatment.
Even if it is in Japan first, it might not be possible for all people to travel there to get the treatment.
This page have been there for a while I'm afraid.Do you know if this a new addition to their webpage?
I know they've had stuff listed on their website for awhile pertaining to this trial but it's been in limbo forever.
It is not, and this twitter post means nothing.Yeah it Seems like its recent, they posted about it on their twitter yesterday
- Reaction score
- 23
This page have been there for a while I'm afraid.
It is not, and this twitter post means nothing.
Oh well... At least its going to be released by shiseido before replicel finishes their clinical trials
Noisette
Established Member
- Reaction score
- 1,341
RepliCel Provides Update on Shiseido License and Co-Development for RCH-01
http://www.cbj.ca/replicel-provides-update-on-shiseido-license-and-co-development-for-rch-01/
http://www.cbj.ca/replicel-provides-update-on-shiseido-license-and-co-development-for-rch-01/
- Reaction score
- 594
So Replicel aren't pulling their own weight on the partnership in developing RCH 01 and are leaving the Japanese partner to do all the research? Doesn't bother me who does the research, as long as it gets done right and gets done on time. Bad news for Western commercialisation but no inactive on the Shissido/Asian branch.
- Reaction score
- 594
As long as it doesn't stop Shiseido I don't care. I always assumed Shiseido, with their superior funding, were doing the lions share of the work anyway.
- Reaction score
- 2,634
As announced in July, RepliCel’s RCH-01 product, for the treatment of androgenetic alopecia, is now under clinical investigation at Tokyo Medical University Hospital and Toho University Ohasi Medical Center, by Drs. Tsuboi and Niiyama.
What does that mean 'under investigation' ?? Did Shiseido finally start Phase II trials in Japan or not?
As long as it doesn't stop Shiseido I don't care. I always assumed Shiseido, with their superior funding, were doing the lions share of the work anyway.
Still, reading that, all I could think was "Damn it guys, keep your heads in the game."
- Reaction score
- 594
Sounds like they've started yeah...What does that mean 'under investigation' ?? Did Shiseido finally start Phase II trials in Japan or not?
- Reaction score
- 594
Still, reading that, all I could think was "Damn it guys, keep your heads in the game."
Yeah. It means that commercialisation outside of Asia is increasingly unlikely, but it shouldn't be a barrier for people willing to travel to Asia.
Desmond_84
Established Member
- Reaction score
- 359
Hi guys. I've been doing alot of digging around but can't find any information about the Shiseido trial. Have they injected the first patient yet? This trial was set to begin in November 2015! Btw isn't it odd that Replicel has stopped updating everyone on the Shiseido progress.
I suppose if noisette hasn't found it, it probably doesn't exist
I suppose if noisette hasn't found it, it probably doesn't exist
Hi guys. I've been doing alot of digging around but can't find any information about the Shiseido trial. Have they injected the first patient yet? This trial was set to begin in November 2015! Btw isn't it odd that Replicel has stopped updating everyone on the Shiseido progress.
I suppose if noisette hasn't found it, it probably doesn't exist
Not really odd if the two companies are butting heads.
If replicel isnt doing their part, you cant really expect them to keep us up to date on shisiedos progress
Noisette
Established Member
- Reaction score
- 1,341
Hi guys. I've been doing alot of digging around but can't find any information about the Shiseido trial. Have they injected the first patient yet? This trial was set to begin in November 2015! Btw isn't it odd that Replicel has stopped updating everyone on the Shiseido progress.
I suppose if noisette hasn't found it, it probably doesn't exist
Hey bro
I don't know if you have seen this link on "NIPH Clinical Trials" (This website can be used to cross-search the content of three national clinical research information registries: The University Hospital Medical Information Network Center , The Japan Medical Association Center, The Japan Pharmaceutical Information Center ).
http://rctportal.niph.go.jp/en/detail?trial_id=UMIN000023343
- Reaction score
- 373
I thought it's a 3-year trial? Read that from our site https://www.hairlosstalk.com/news/new-research/shiseido-stem-cell-regeneration/Nice! So we should have the (interim?) results end of next year or begin 2018.
Man I really hope this works so we can fly over to Japan and get a shot of this in 2018.
(Sorry if I'm outdated and fingers crossed for them)
- Reaction score
- 1,332
Yeah I wrote that actually lol.
The information about the 3 year trial was at this page; http://web.archive.org/web/20160630001833/http://the-japan-news.com/news/article/0003046120
I'm not actually 100% sure if that is correct now though?
What is possible too maybe is that they will look at three years at the results of those patients (if the 1 year result is positive) but we will get access after the 1 year. The newly japan regulatory approval process works like this;
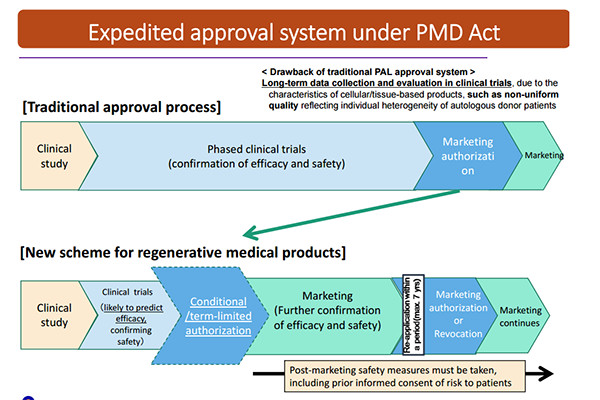
So basically what that would mean is if the 1 year trials is seen as positive and safe they would conditionally approve it so it's accessible to people and then in that conditional approval they still would look at the results and if all is positive they would make the final decision to bring it to the market.
In any case Replicel mentioned multiple times that a 2018 Shiseido release for patients is possible if the outcome is good so yeah .
.
The information about the 3 year trial was at this page; http://web.archive.org/web/20160630001833/http://the-japan-news.com/news/article/0003046120
The clinical study will target about 60 men and women. Root sheath cells will be removed from patients at the Tokyo Medical University Hospital and the Toho University Ohashi Medical Center. Then these cells will be moved to a facility at Shiseido, where they will be cultured. Later, the cultured cells will be returned to the two medical institutions, where they will be transplanted.
The research team will spend three years working to determine whether the patients will be able to regain lost hair.
I'm not actually 100% sure if that is correct now though?
What is possible too maybe is that they will look at three years at the results of those patients (if the 1 year result is positive) but we will get access after the 1 year. The newly japan regulatory approval process works like this;

So basically what that would mean is if the 1 year trials is seen as positive and safe they would conditionally approve it so it's accessible to people and then in that conditional approval they still would look at the results and if all is positive they would make the final decision to bring it to the market.
In any case Replicel mentioned multiple times that a 2018 Shiseido release for patients is possible if the outcome is good so yeah
- Reaction score
- 129
I don't trust Replicel in general. Too much past & present BS going on with them.
I honestly hope they fail to deliver on their agreement with Shiseido and end up forfeiting all the distribution rights to them.
KO1
Established Member
- Reaction score
- 101
Agreed. Their whole concept for existing was that they started calling DS cells of a certain region by a different name that nobody else uses ("DSC cells"). There's no reason to believe this B team going to crack this code that the Aderans A team failed at.I don't trust Replicel in general. Too much past & present BS going on with them.
Also their results have been underwhelming. Aaaaand....what kind of a legitimate company pays money to pump up their stock? lulz
Last edited:
