Bump for greatness. You still around
@InBeforeTheCure? Miss your posts

.
Also as others have already linked this study:
http://journals.plos.org/plosgenetics/article?id=10.1371/journal.pgen.1006594.
Cool.

They also included an analysis with something called DEPICT which wasn't in the preprint (Supplementary Table S5). This tests for pathway enrichment in genes near GWAS hits. Some interesting categories: GLI3 subnetwork and KEGG_MTOR_SIGNALING_PATHWAY, both in the top 10. RUNX2 subnetwork, KEGG_PROSTATE_CANCER, TWIST1 subnetwork, SMAD2 subnetwork, REACTOME_PIP3_ACTIVATES_AKT_SIGNALING, REACTOME_PI3KAKT_ACTIVATION, SMAD3 subnetwork, and SMAD1 subnetwork. Also various categories involved in transcriptional regulation, and in the paper they also mention multiple genes involved in the Wnt pathway.
The million dollar question though is: How do all these components interact with each other and with androgens to cause balding? Maybe we can look for
some hints in Chew et al.'s microarray study. Here's an example...
Twist1 (and also Twist2 obviously) is a basic helix-loop-helix (bHLH) transcription factor. To bind DNA, bHLH transcription factors typically (maybe always?) need to dimerize with other bHLH proteins. So Twist1 can dimerize with another Twist1 protein (Twist1 homodimer) or it can dimerize with E-proteins (which are also bHLH) such as Tcf3 or Tcf12 (Twist1 heterodimer), and also it can dimerize with things like Hand1 and Hand2. Regulation of gene expression by Twist differs depending on its dimer composition; for example, Twist1 heterodimers downregulate FGFR2 in cranial sutures while Twist1 homodimers upregulate FGFR2. Twist1 and Tcf12 are both GWAS hits for A.G.A, so Twist1 dimer composition is probably important for us. Twist1 is also the most common mutation in Saethre-Chotzen syndrome (SCS), characterized by premature closing of the cranial sutures (Twist1 heterodimers keep these sutures open), and Tcf12 mutations are also known to cause SCS.
Now, one way that cells can control Twist1 dimer composition is through regulation of Inhibitor of DNA Binding expression (ID1, ID2, ID3, and ID4), which are helix-loop-helix (HLH) proteins that lack the basic domain and can't bind DNA. ID proteins compete with bHLH TFs for E-proteins, so when ID expression is low comparatively more Twist heterodimers will be formed, and when ID expression more Twist homodimers will be formed.
OK, that's hopefully enough background. So look what happens to ID1, ID2, and ID3 expression in dermal papilla cells with exposure to DHT. The top left graph is in balding DPCs treated with 10nM DHT, the top right is balding DPCs treated with 1nM DHT, the bottom left is in non-balding DPCs treated with 10nM DHT, and the bottom right is in non-balding DPCs treated with 1nM DHT. Notice how in all four cases, ID1 and ID2 spike
rapidly after treatment with DHT within 15 minutes. ID3 also spikes rapidly in the non-balding cells, but in the balding cells it lags a bit for some reason. Then, after a few hours, ID expression returns to normal.
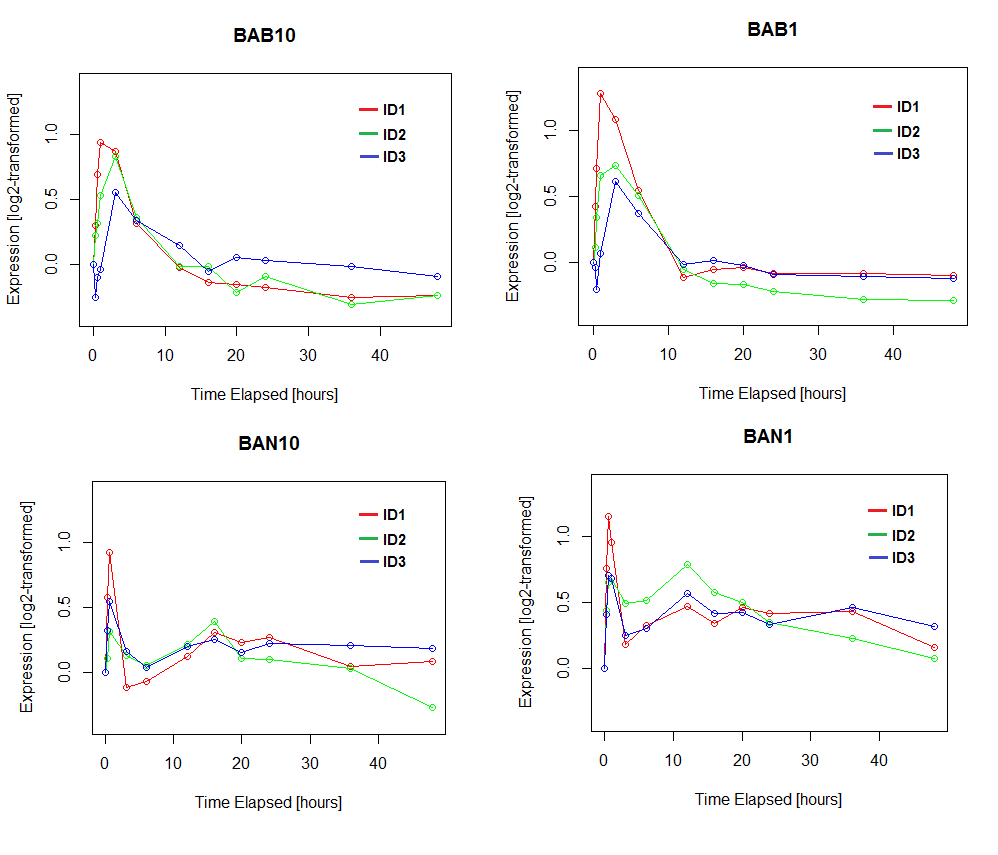
The spike in ID expression happens within 15 minutes, which is probably too fast to be the result of classical genomic regulation by AR. Instead, this would be a response to non-genomic AR signaling. IDs are canonical target genes of SMADs, and indeed, if you take the list of genes upregulated at the 30 minute mark and analyze them with TFacts, you'll find that they're mostly enriched for SMAD target genes. Therefore, the most likely explanation for the spike in ID expression is the known upregulation of TGF-beta1 by DHT. The timing is also right -- that one Philpott study found the induction of TGF-beta1 by DHT to be less than an hour, so they concluded that this was probably the result of non-genomic AR signaling. The drop in ID expression after a few hours, when AR would be predominantly nuclear, is also consistent with this interpretation. The one problem is that Chew's microarray didn't detect TGFB1 expression
at all, so maybe their probeset didn't detect it for some reason?
But anyway, here you have a possible connection between some of the GWAS hits -- SRD5A2, AR, TGFB1, TWIST1, TWIST2, and TCF12. Runx2 and Runx3, which bind SMADs, are also major downstream modulators of TGF-beta. So probably both expression levels of the upstream components of this pathway as well as their downstream modulators can give different "outputs" when DPCs are exposed to androgens. Also, given that Twist1 knockout mice
stay in anagen forever(?), Twist1 must play a very important but unexplored role in hair follicle biology.
What do you think?
I wish
@InBeforeTheCure was as active as the shitposters that flood the forum every day. Or at least as active as 0.1% of the average posting rate of the ''
impact session''
Thanks. But come on, the impact section is glorious.