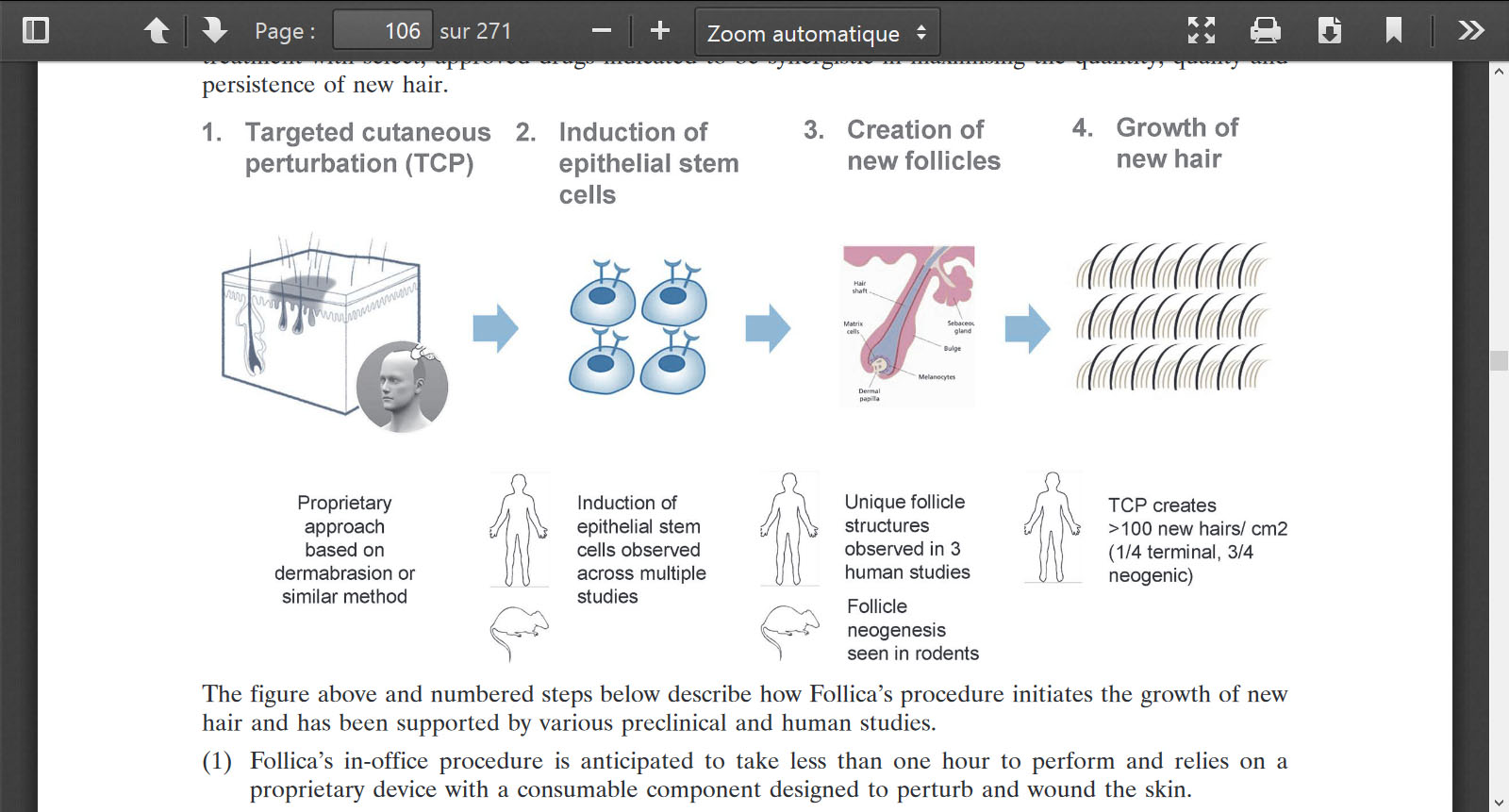
Mice again.

Not only on Mice  Both, Mice and Humans. They have completed 3 clinical studies
Both, Mice and Humans. They have completed 3 clinical studies
and Olle said “all of which confirm that we can consistently create new hair follicles in mice and in humans. As far as I know, no other approach has been able to achieve that.”
And " Based on previous experiments, Follica believes that it has several potential drug candidates to add to TCP to enrich for terminal hair. Follica’s hypothesis was further strengthened when a third party academic group, working independently of Follica, published positive results using a form of TCP and one drug Follica plans on adding to TCP in the future. The company believes that the work that was done by the third party group was covered under its filed intellectual property (please see below table and discussion of intellectual property).
Lithium 8% already failed. It said so in the forst press release.
- - - Updated - -
And Noisette, does this mean the procedure is only for people with very early hair loss? What constitutes early hair loss? NW2-3-4?
I think that is also for high norwood like 5. Because on an old patent (clinal trial phase IIa) they said they have test it on Norwood 3, 4 and 5
So their protocol would be enhanced since this clinical study 
"
8.1 STUDY PROTOCOL
[00637]
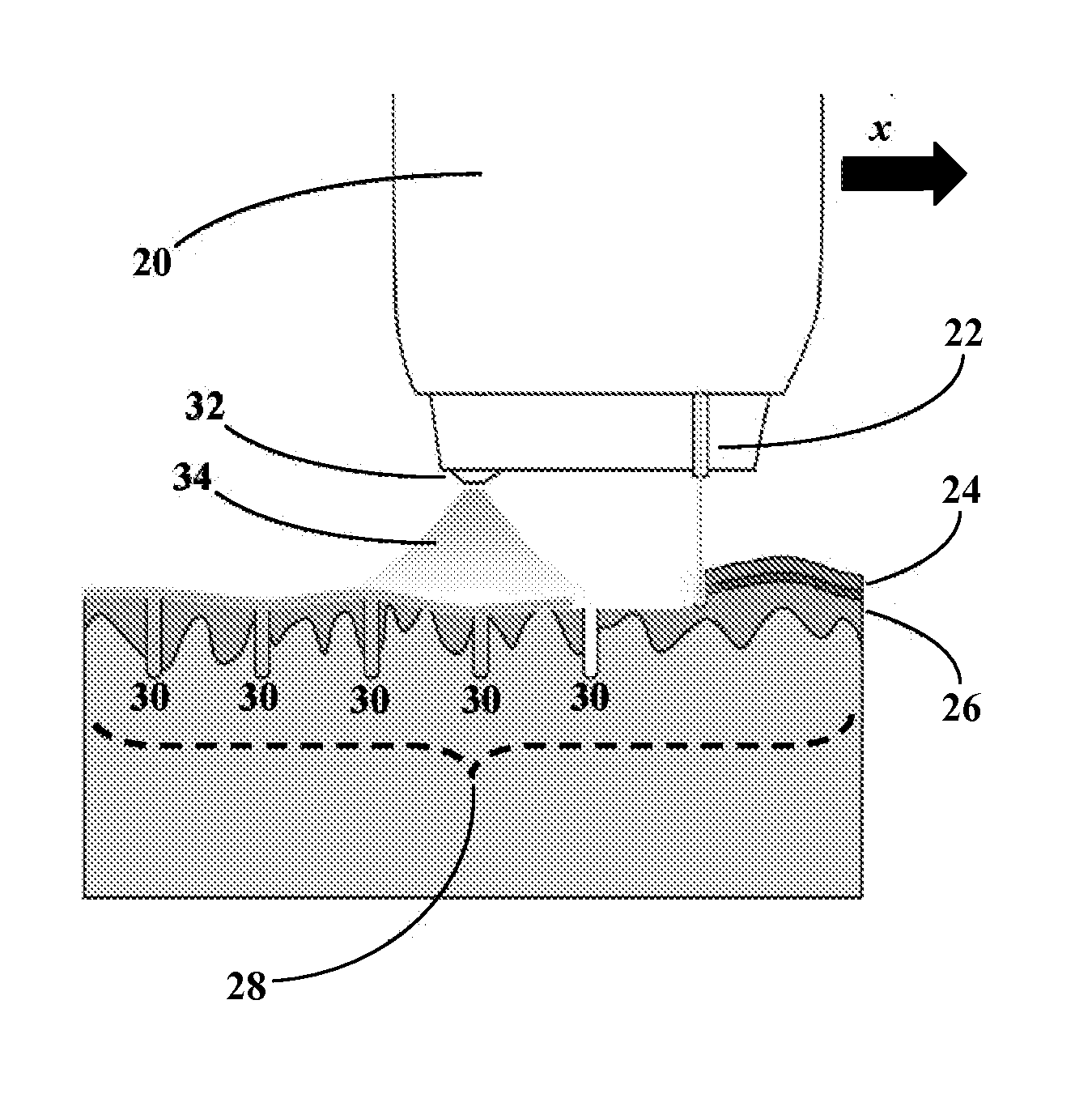
The following protocol describes a Phase Ila clinical study that evaluated the effect of dermabrasion and post-perturbation treatment with a hydrogel (comprising Carbomer (Carbopol 980), glycerol, sodium hydroxide, methylparaben, propylparaben, and purified water) on (i) the formation of neogenic-like or activated or stimulated hair follicle structures (e.g., NL, PEL and PELA as described in Section 5.8.4.1 supra) and (ii) hair growth.
[00638] Diagnosis and main criteria for inclusion are Caucasian males 20-65 years of age, providing written informed consent, who have androgenetic alopecia with the presence of a vertex transition zone defined as an area possessing both terminal and miniaturized hairs, Hamilton-
Norwood type 3V, 4, 5, 5A, or 5V, with a vertex area large enough to accommodate both treatment sites, and Fitzpatrick skin type 1-4. "
"[00658] In dermabraded areas of skin, induction of neogenic-like hair follicles and activated, stimulated, or reorganized pre-existing (including vellus-sized) follicles was measured as detected and analyzed by skin biopsy analysis at Day 14. In contrast to what is generally found in unwounded scalp skin,the controlled perturbation in this study 1) induced neogenic-like follicles and 2) placed preexisting follicles into a reorganized and activated state. The structures of interest detected and counted in the Day 14 biopsies are observed only rarely in unwounded skin. Therefore, they are comprised of neogenic-like and activated, stimulated, or reorganized structures "
[00656] It was found that, for subjects treated with dermabrasion plus hydrogel:
• From baseline to Day 84 (3 months), target area hair counts (also referred to as "TAHC") of all
hair show substantial increases that are statistically significant (see Table 9). This change in counts of all hair from baseline is maintained through the last day of measurement, Day 168 (6 months; See Table 10) and the change is statistically significant on Day 168.
s
ource: https://www.google.com/patents/WO20...ved=0ahUKEwiQuLjq3-DMAhWThRoKHabpA4cQ6AEIMzAD