Noisette
Established Member
- Reaction score
- 1,341
Hey girls and guys
I think it's the patent which was mentioned in the article called " Follica Announces Allowance for a U.S. Patent Related to its Principal Technology Platform to Treat Hair Loss ". In september 2015, Puretech Health has announced that it has received notice from the US Patent and Trademark Office of a patent allowance on its novel hair loss treatments.
- The new patent published at the end of March 2017 is untitled " Needling device and drug applicator "
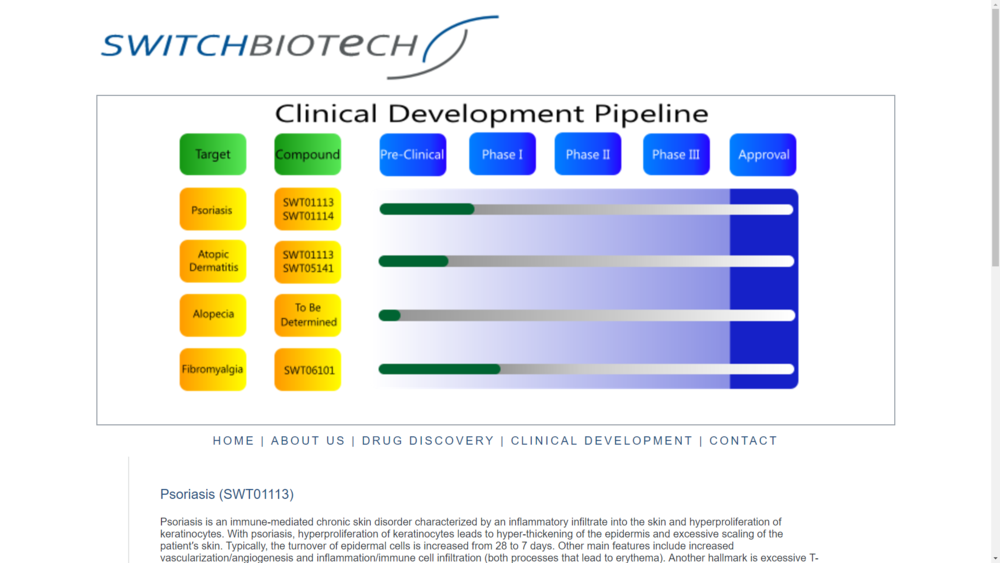
1. In this we can read that the applicators may also be used in combination with drugs for alopecia being developed by SWITCH Biotech LLC. There is a developement of an unknown new drug (pre-clinical phase).
2. May also be used in combination with SM04554, and KYTH105 (setipiprant) !
Source: https://patentscope.wipo.int/search/en/detail.jsf?docId=WO2017054009
I paste some informations. Sorry for the long post

Summary : A needling device may be used for needling of a subject's skin, and a drug applicator device may be used for applying a drug to a subject's skin. For example, a needling device may be applied to a subject's skin for hair growth applications, or may be used for wrinkle, scar, or tattoo removal. A drug applicator device may be used for multiple drug application purposes such as applying a hair growth compound to the skin of a subject.
2. BACKGROUND OF THE INVENTION
2.1 FIELD OF THE INVENTION
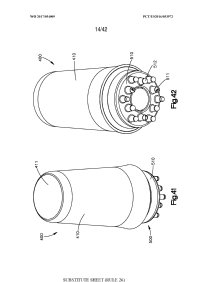
[0002] The following description relates to a needling device for needling of a subject's skin by a user such as a physician or any user, and a fluid or drug dispensing applicator device for massaging and applying a fluid or drug to a subject's skin. In certain embodiments, the subject is in need of inducing hair growth or hair follicle neogenesis, is need of preventing hair loss, and/or is a subject as described in Section 5.5. For example, a needling device may be applied to a subject's skin for hair growth applications, or may also be used for wrinkle reduction, scar revision, hair removal, tattoo removal, and pigmentation. The fluid or drug applicator device may be used for multiple drug application purposes such as applying a hair growth compound to the skin of a subject or treatment for other dermatological conditions.
[00010] In yet another additional aspect, a method of using a needling device and a drug applicator for stimulating hair growth includes providing a needling device including a sheath assembly having a needle array and a main unit having a motor for driving the needle array, providing a drug applicator for drug delivery and massaging including a housing, a drug delivery cartridge carried by the housing, a massage head which is mounted on the housing for massaging a subject's skin, using the needling device to perform targeted cutaneous perturbation for disrupting a layer of a human scalp, after using the needling device, using the drug applicator for applying a drug to the disrupted area of the human scalp. In a specific embodiment, the disrupted layer of the human scalp is the basal or suprabasal epidermal layer, and the drug that is applied is minoxidil or a proteasome inhibitor including lactacystin, a peptidyl aldehyde, or pentoxyfilline (PTX). In a specific embodiment, the method further comprises delivering the drug directly to the human scalp to facilitate transdermal transport. In a specific embodiment, the method further comprises massaging the drug that is delivered to the human scalp to evenly spread the drug.
(...) the applicator is configured to stretch or massage a subject's skin while simultaneously dispensing the drug. In a specific embodiment, an amount of drug being dispensed can be controlled by controlling a number of massaging strokes per massaging cycle, a stroke distance per massaging cycle, or a profile of the dispensing mechanism. In a specific embodiment, the drug that is applied is minoxidil or a proteasome inhibitor including lactacystin, a peptidyl aldehyde, or pentoxyfilline (PTX).
(...) In a specific embodiment, the applicator further comprises a keypad positioned on a surface of the applicator. In a specific embodiment, the applicator further comprises an LED and a control unit configured to operate the LED in response to determining that a next dose is due. In a specific embodiment, the applicator further comprises an inductive charging base configured to receive the housing for charging the applicator. In a specific embodiment, the applicator further comprises a light for illuminating a target region of the user's skin.
(...) a cam drive which is mounted in the housing and rotates the drug delivery cartridge and the massage head for providing a massaging effect, and a controller and sensors that are configured to detect a rotation angle of the massage head to determine a dispensed dosage using the detected angle<;
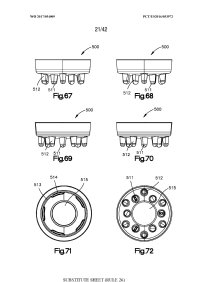
(...) [000138] In an example, density control of the number of perturbations or skin perforations per square area of the target surface is available in a low density, medium density, and high density configuration for ease and simplicity to a user. The density of needling referred to herein is determined by the number of needles 216 in contact with a subject's skin at a given time during a needling operation and per square area of the subject's skin. The density may range in value and in an example the density ranges in value from the ratio of skin perforations surface area to the total skin surface area being 1% to that ratio being 40%.
5.1.2 ASPECTS OF THE DESCRIBED EMBODIMENTS OF THE NEEDLING
DEVICE [000161] In an aspect, the needling devices and needling adaptors described in all embodiments above suitable to promote hair follicle neogenesis through targeted cutaneous perturbation (TCP) of the skin (e.g., the scalp). In a specific embodiment, TCP refers to integumental perturbation of one or more epidermal layers (see, e.g., Sections 5.3 and 5.4), for example, the basal and/or suprabasal epidermal layers. In a specific embodiment, the integumental perturbation performed by the needling devices and needling adaptors described herein is followed by the application of a compound (see, e.g., Section 5.6) to the skin. Without being bound by any particular theory, TCP triggers generation of new hair follicles by induction of epithelial stem cells. See, e.g., Sections 5.3 and 5.4 for a more detailed discussion of integumental perturbation and methods of using the needling devices and needling adaptors. In other examples, the needling devices and needling adaptors or interchangeable needling adaptors with needle arrays having different configurations (such as is illustrated in the example of FIGS. 18 and 24) can be used for other applications such as wrinkle reduction, scar revision, hair removal, and tattoo removal.
(...) The needling device may also be used in combination with guides that fit over the needle array in order to direct movement through a subject's hair; for example, the guides can be similar to hair clipper attachments.
(...) In an example, the fluid applicator is a drug applicator that is used for the application of minoxidil to a subject's scalp or for a variety of different applications using different chemicals. With respect to all embodiments of the drug or fluid applicators, it should be appreciated that any type of fluid may be used and this invention is not limited to the use of a drug.
[000169] In one aspect, drug applicators may be used in combination with other agents or treatments that stimulate hair growth.
In another aspect, the applicators may also be used in combination with drugs for alopecia being developed by SWITCH Biotech LLC.
[000170] In addition, other compounds that may be used with the applicator include compounds identified by the following company and company ID, Actelion ACT- 129968 (setipiprant), Actimis AP768, Amira AM211, Amira AM461, Amgen AMG853, Array BiPharma ARRY-502, AstraZeneca AZD1981, AstraZeneca AZD8075, AstraZeneca AZD5985, Merck MK-7246, Novartis QAV680, Oxagen OC000459, Ocagen OC002417, Pulmagen ADC3680B, Shionogi S-555739, BBI-5000, SM04554, and KYTH105 (setipiprant).
[000254] In a specific embodiment, the devices and/or mobile apps described herein are suitable to achieve one or more of the following nonlimiting examples of biological outcomes in a subject on which the devices and/or mobile apps were used: to promote generation of new hair follicles ("follicle neogenesis"); to promote formation of neogenic-like (NL) follicular structures; to promote activation (possibly by reorganization) of existing hair follicles; to promote formation of pre-exi sting-like (PEL) or pre-exi sting-like, attached (PELA) follicular structures; to promote development of hair follicles, for example, to promote the growth of terminal hair (in preference to vellus hair); to promote the branching of pre-existing hair follicles (seen as an increased number of hair shafts per pore); to increase the width of hair follicles (thereby promoting growth of an increased shaft width); to delay or prevent follicle senescence;
[000263] The subject may have a disease or disorder of balding or hair loss (including hair thinning), such as forms of nonscarring (noncicatricial) alopecia, such as androgenetic alopecia (Androgenetic Alopecia), including male-patterned hair loss (MPHL) or female-patterned hair loss (FPHL) (e.g., thinning of the hair, i.e., diffuse hair loss in the frontal/parietal scalp), or any other form of hair loss caused by androgens, toxic alopecia, alopecia areata (including alopecia universalis), scarring (cicatricial) alopecia, pathologic alopecia (caused by, e.g., medication, trauma stress, autoimmune diseases, malnutrition, or endocrine dysfunction), trichotillomania, a form of hypotrichosis, such as congenital hypotrichosis, or lichen planopilaris, or any other condition of hair loss or balding known in the art or described infra.
I think it's the patent which was mentioned in the article called " Follica Announces Allowance for a U.S. Patent Related to its Principal Technology Platform to Treat Hair Loss ". In september 2015, Puretech Health has announced that it has received notice from the US Patent and Trademark Office of a patent allowance on its novel hair loss treatments.
- The new patent published at the end of March 2017 is untitled " Needling device and drug applicator "
1. In this we can read that the applicators may also be used in combination with drugs for alopecia being developed by SWITCH Biotech LLC. There is a developement of an unknown new drug (pre-clinical phase).
2. May also be used in combination with SM04554, and KYTH105 (setipiprant) !
Source: https://patentscope.wipo.int/search/en/detail.jsf?docId=WO2017054009
I paste some informations. Sorry for the long post
Summary : A needling device may be used for needling of a subject's skin, and a drug applicator device may be used for applying a drug to a subject's skin. For example, a needling device may be applied to a subject's skin for hair growth applications, or may be used for wrinkle, scar, or tattoo removal. A drug applicator device may be used for multiple drug application purposes such as applying a hair growth compound to the skin of a subject.
2. BACKGROUND OF THE INVENTION
2.1 FIELD OF THE INVENTION
[0002] The following description relates to a needling device for needling of a subject's skin by a user such as a physician or any user, and a fluid or drug dispensing applicator device for massaging and applying a fluid or drug to a subject's skin. In certain embodiments, the subject is in need of inducing hair growth or hair follicle neogenesis, is need of preventing hair loss, and/or is a subject as described in Section 5.5. For example, a needling device may be applied to a subject's skin for hair growth applications, or may also be used for wrinkle reduction, scar revision, hair removal, tattoo removal, and pigmentation. The fluid or drug applicator device may be used for multiple drug application purposes such as applying a hair growth compound to the skin of a subject or treatment for other dermatological conditions.
[00010] In yet another additional aspect, a method of using a needling device and a drug applicator for stimulating hair growth includes providing a needling device including a sheath assembly having a needle array and a main unit having a motor for driving the needle array, providing a drug applicator for drug delivery and massaging including a housing, a drug delivery cartridge carried by the housing, a massage head which is mounted on the housing for massaging a subject's skin, using the needling device to perform targeted cutaneous perturbation for disrupting a layer of a human scalp, after using the needling device, using the drug applicator for applying a drug to the disrupted area of the human scalp. In a specific embodiment, the disrupted layer of the human scalp is the basal or suprabasal epidermal layer, and the drug that is applied is minoxidil or a proteasome inhibitor including lactacystin, a peptidyl aldehyde, or pentoxyfilline (PTX). In a specific embodiment, the method further comprises delivering the drug directly to the human scalp to facilitate transdermal transport. In a specific embodiment, the method further comprises massaging the drug that is delivered to the human scalp to evenly spread the drug.
(...) the applicator is configured to stretch or massage a subject's skin while simultaneously dispensing the drug. In a specific embodiment, an amount of drug being dispensed can be controlled by controlling a number of massaging strokes per massaging cycle, a stroke distance per massaging cycle, or a profile of the dispensing mechanism. In a specific embodiment, the drug that is applied is minoxidil or a proteasome inhibitor including lactacystin, a peptidyl aldehyde, or pentoxyfilline (PTX).
(...) In a specific embodiment, the applicator further comprises a keypad positioned on a surface of the applicator. In a specific embodiment, the applicator further comprises an LED and a control unit configured to operate the LED in response to determining that a next dose is due. In a specific embodiment, the applicator further comprises an inductive charging base configured to receive the housing for charging the applicator. In a specific embodiment, the applicator further comprises a light for illuminating a target region of the user's skin.
(...) a cam drive which is mounted in the housing and rotates the drug delivery cartridge and the massage head for providing a massaging effect, and a controller and sensors that are configured to detect a rotation angle of the massage head to determine a dispensed dosage using the detected angle<;
(...) [000138] In an example, density control of the number of perturbations or skin perforations per square area of the target surface is available in a low density, medium density, and high density configuration for ease and simplicity to a user. The density of needling referred to herein is determined by the number of needles 216 in contact with a subject's skin at a given time during a needling operation and per square area of the subject's skin. The density may range in value and in an example the density ranges in value from the ratio of skin perforations surface area to the total skin surface area being 1% to that ratio being 40%.
5.1.2 ASPECTS OF THE DESCRIBED EMBODIMENTS OF THE NEEDLING
DEVICE [000161] In an aspect, the needling devices and needling adaptors described in all embodiments above suitable to promote hair follicle neogenesis through targeted cutaneous perturbation (TCP) of the skin (e.g., the scalp). In a specific embodiment, TCP refers to integumental perturbation of one or more epidermal layers (see, e.g., Sections 5.3 and 5.4), for example, the basal and/or suprabasal epidermal layers. In a specific embodiment, the integumental perturbation performed by the needling devices and needling adaptors described herein is followed by the application of a compound (see, e.g., Section 5.6) to the skin. Without being bound by any particular theory, TCP triggers generation of new hair follicles by induction of epithelial stem cells. See, e.g., Sections 5.3 and 5.4 for a more detailed discussion of integumental perturbation and methods of using the needling devices and needling adaptors. In other examples, the needling devices and needling adaptors or interchangeable needling adaptors with needle arrays having different configurations (such as is illustrated in the example of FIGS. 18 and 24) can be used for other applications such as wrinkle reduction, scar revision, hair removal, and tattoo removal.
(...) The needling device may also be used in combination with guides that fit over the needle array in order to direct movement through a subject's hair; for example, the guides can be similar to hair clipper attachments.
(...) In an example, the fluid applicator is a drug applicator that is used for the application of minoxidil to a subject's scalp or for a variety of different applications using different chemicals. With respect to all embodiments of the drug or fluid applicators, it should be appreciated that any type of fluid may be used and this invention is not limited to the use of a drug.
[000169] In one aspect, drug applicators may be used in combination with other agents or treatments that stimulate hair growth.
In another aspect, the applicators may also be used in combination with drugs for alopecia being developed by SWITCH Biotech LLC.
[000170] In addition, other compounds that may be used with the applicator include compounds identified by the following company and company ID, Actelion ACT- 129968 (setipiprant), Actimis AP768, Amira AM211, Amira AM461, Amgen AMG853, Array BiPharma ARRY-502, AstraZeneca AZD1981, AstraZeneca AZD8075, AstraZeneca AZD5985, Merck MK-7246, Novartis QAV680, Oxagen OC000459, Ocagen OC002417, Pulmagen ADC3680B, Shionogi S-555739, BBI-5000, SM04554, and KYTH105 (setipiprant).
[000254] In a specific embodiment, the devices and/or mobile apps described herein are suitable to achieve one or more of the following nonlimiting examples of biological outcomes in a subject on which the devices and/or mobile apps were used: to promote generation of new hair follicles ("follicle neogenesis"); to promote formation of neogenic-like (NL) follicular structures; to promote activation (possibly by reorganization) of existing hair follicles; to promote formation of pre-exi sting-like (PEL) or pre-exi sting-like, attached (PELA) follicular structures; to promote development of hair follicles, for example, to promote the growth of terminal hair (in preference to vellus hair); to promote the branching of pre-existing hair follicles (seen as an increased number of hair shafts per pore); to increase the width of hair follicles (thereby promoting growth of an increased shaft width); to delay or prevent follicle senescence;
[000263] The subject may have a disease or disorder of balding or hair loss (including hair thinning), such as forms of nonscarring (noncicatricial) alopecia, such as androgenetic alopecia (Androgenetic Alopecia), including male-patterned hair loss (MPHL) or female-patterned hair loss (FPHL) (e.g., thinning of the hair, i.e., diffuse hair loss in the frontal/parietal scalp), or any other form of hair loss caused by androgens, toxic alopecia, alopecia areata (including alopecia universalis), scarring (cicatricial) alopecia, pathologic alopecia (caused by, e.g., medication, trauma stress, autoimmune diseases, malnutrition, or endocrine dysfunction), trichotillomania, a form of hypotrichosis, such as congenital hypotrichosis, or lichen planopilaris, or any other condition of hair loss or balding known in the art or described infra.
Last edited:
