Noisette
Established Member
- Reaction score
- 1,341
Hi ladies and gentlemen,
After a few months, I came here just to say hello with some little news..
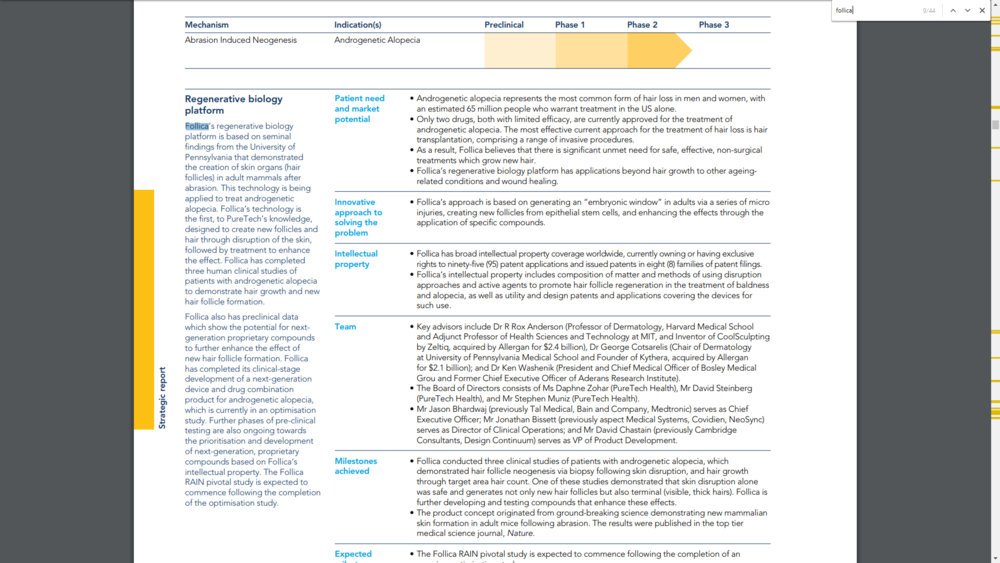
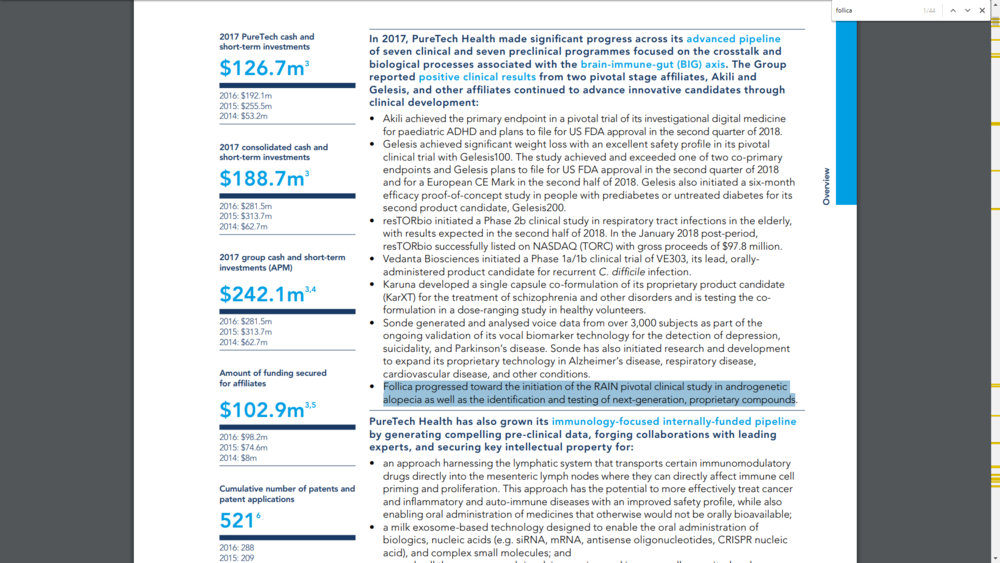
That's not really new but we can see that " Follica has completed its clinical-stage development of a next-generation device and drug combination product for androgenetic alopecia, which is currently in an optimisation study. Further phases of pre-clinical testing are also ongoing towards the prioritisation and development of next-generation, proprietary compounds based on Follica’s intellectual property. The Follica RAIN pivotal study is expected to commence following the completion of the optimisation study."
So
1. Development of next generation device and Drug combination product done.
2. Is currently in an optimisation study.
3. Ongoing pre-clinical testing towards the prioritisation and develoment of next generation compounds.
4. Pivotal study following the completion of the ongoing optimisation study.
Source: http://puretechhealth.com/images/investors/PureTech-Health-ARA-2017_optimized-for-printing.pdf
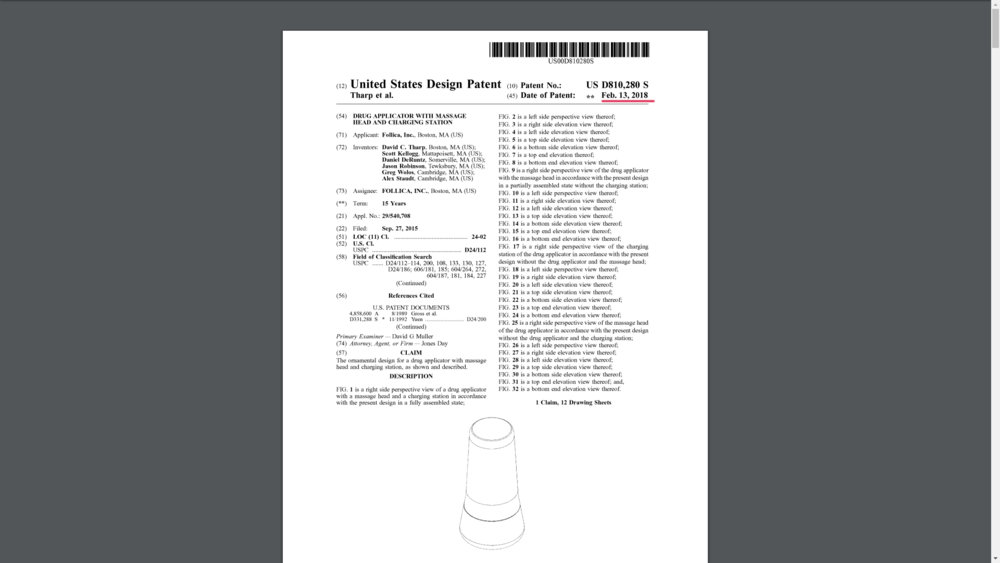
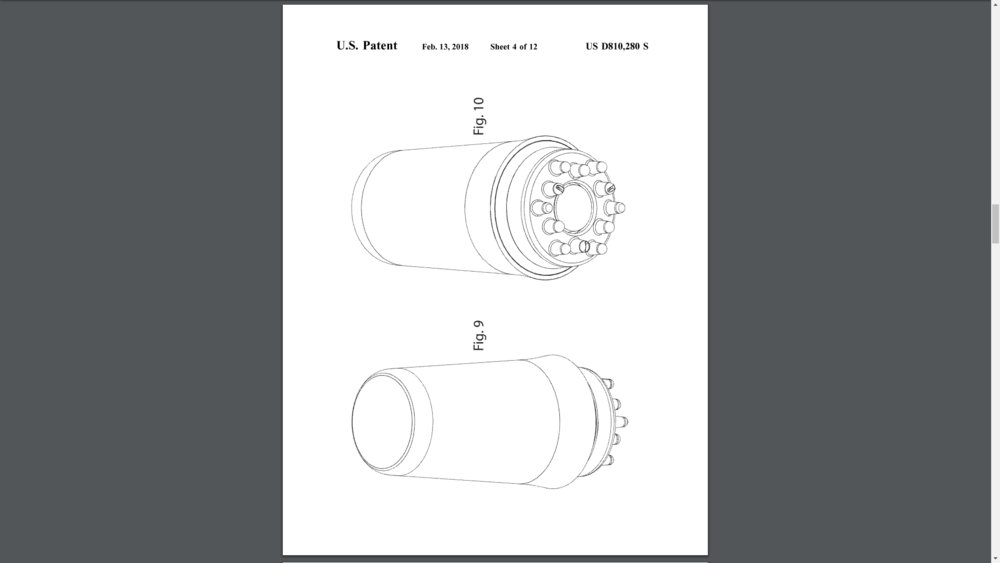
And in February 13th 2018, Follica has obtained a granted patent
Source: https://patentimages.storage.googleapis.com/be/0a/6b/f3cb333585bc13/USD810280.pdf
May the force be with your scalp
After a few months, I came here just to say hello with some little news..
That's not really new but we can see that " Follica has completed its clinical-stage development of a next-generation device and drug combination product for androgenetic alopecia, which is currently in an optimisation study. Further phases of pre-clinical testing are also ongoing towards the prioritisation and development of next-generation, proprietary compounds based on Follica’s intellectual property. The Follica RAIN pivotal study is expected to commence following the completion of the optimisation study."
So
1. Development of next generation device and Drug combination product done.
2. Is currently in an optimisation study.
3. Ongoing pre-clinical testing towards the prioritisation and develoment of next generation compounds.
4. Pivotal study following the completion of the ongoing optimisation study.
Source: http://puretechhealth.com/images/investors/PureTech-Health-ARA-2017_optimized-for-printing.pdf
And in February 13th 2018, Follica has obtained a granted patent
Source: https://patentimages.storage.googleapis.com/be/0a/6b/f3cb333585bc13/USD810280.pdf
May the force be with your scalp
Last edited:
