- Reaction score
- 146
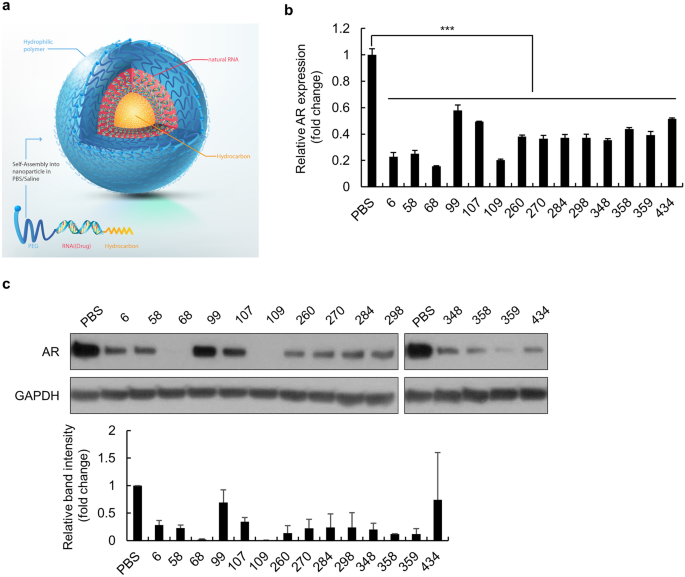
Weekly treatment with SAMiRNA targeting the androgen receptor ameliorates androgenetic alopecia - Scientific Reports
Androgenetic alopecia (Androgenetic Alopecia) is the most common type of hair loss in men and women. Dihydrotestosterone (DHT) and androgen receptor (AR) levels are increased in patients with Androgenetic Alopecia, and DHT-AR signaling correlates strongly with Androgenetic Alopecia pathogenesis. In this study, treatment with self-assembled micelle...
In the high-dose (5 mg/ml) clinical study, AR68 was given once per week for 24 weeks and showed 83% efficacy in increasing hair counts compared with finasteride. No side effects were observed. Therefore, SAMiRNA targeting AR mRNA is a potential novel topical treatment for Androgenetic Alopecia.


