You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
Lots of New Info on Follica
- Thread starter Xaser94
- Start date
- Reaction score
- 2,634
I think some are confusing the 1.5mm study to what cots was taking about. 2 completely different theories. What size does prettyfly use? I know he was using .5 starting out.
+1 on the derminator
He's using a 1.5mm dermaroller.
- - - Updated - - -
Hellouser,
Do you use the dermastamp?? In which ways do you think it's better than a dermaroller? I am considering getting one. I love dermarolling and feel that I've had some results with it, however, also feel like it may be causing too much tissue damage, with the back and forth rolling motion.
I use a dermaroller atm. Dermartamp just hurts a lot less. This is the main difference though:
- Reaction score
- 214
Theoretically this should work on the nape of the neck, right? I have retrograde alopecia and it'd be fantastic if I could fill that area in.
KO1
Established Member
- Reaction score
- 101
I have seen the data of minoxidil also together with the lithium gel from Follica released from the 2nd clinical trial (dermabrasion + compound + hydrogel). The PDF was up a few years ago. The minoxidil did work better, but only very slightly. The lithium gel was nothing special either as they actually do mention in the article.
Follica has actually never released the effects of Lithium Gluconate 8% after wounding. I do recall the data you refer to, but that was just the impact of wounding on EDIHN - the control group. No matter though as in either case Lithium didn't work.
Wondering what the new compound is though...Could be Seti, could be VPA, could be PGE2...
My theory is that Cots started focusing on PGD2 as an inhibitor of EDIHN after Phase 2 trials failed, which led to his work on that molecule. That said overall we know that open, large surface area wounding grows hair, and things like Wnt, PGE2, and FGF promote it while PGD2 stops it. Nobody has yet tried applying all these things.
For those of us who are ready to take sandpaper to their heads. A heads up - the idea is to create a wound with a large surface area, something that can't close in on itself, so not dermarolling. Dermabrasion is a good way to do it, but to do it, you need to be under anasthesia, it is an outpatient surgical procedure. Chemical peels are also another option.
- Reaction score
- 2,634
If anyone wants to work at Follica, get your resumes ready:
http://puretechhealth.com/careers.php?gh_jid=189240
http://puretechhealth.com/careers.php?gh_jid=189236
http://puretechhealth.com/careers.php?gh_jid=189240
http://puretechhealth.com/careers.php?gh_jid=189236
- Reaction score
- 2,634
Those dermarolling/minoxidil results are amazing, do you know how frequently he derma rolls? I've heard people doing it everyday to doing it once a week, any evidence on what is most effective?
Once a week.
Xaser94
Established Member
- Reaction score
- 661
If anyone wants to work at Follica, get your resumes ready:
http://puretechhealth.com/careers.php?gh_jid=189240
http://puretechhealth.com/careers.php?gh_jid=189236
The fact its for product development has got me excited. Id be very disappointed if its all this for wounding plus minoxidil. Not the worst thing that could happen, but Id really like to see a new compound in the mix. Its funny to think about how much George Cotsarelis was trolling desmond. Was he being semi truthful when he was comparing a partial cure to a full cure taking way more money and lots more time or if it was just to troll. Imagine he has an account on the boards and is just getting a kick out of all this.
Follisket
Established Member
- Reaction score
- 288
If anyone wants to work at Follica, get your resumes ready:
http://puretechhealth.com/careers.php?gh_jid=189240
http://puretechhealth.com/careers.php?gh_jid=189236

Balding brethren, prepare to infiltrate.
Like the movie catch me if you can?
- - - Updated - - -
Do you know if anyone is using an activator? Watch Cooley do prp to Tillman and Cooley mentioned adding an activator. Calcium thrombin. Would be interesting if that would help after DM. Doesn't Swiss mention something?
- - - Updated - - -
He's using a 1.5mm dermaroller.
- - - Updated - - -
I use a dermaroller atm. Dermartamp just hurts a lot less. This is the main difference though:

Do you know if anyone is using an activator? Watch Cooley do prp to Tillman and Cooley mentioned adding an activator. Calcium thrombin. Would be interesting if that would help after DM. Doesn't Swiss mention something?
Noisette
Established Member
- Reaction score
- 1,341
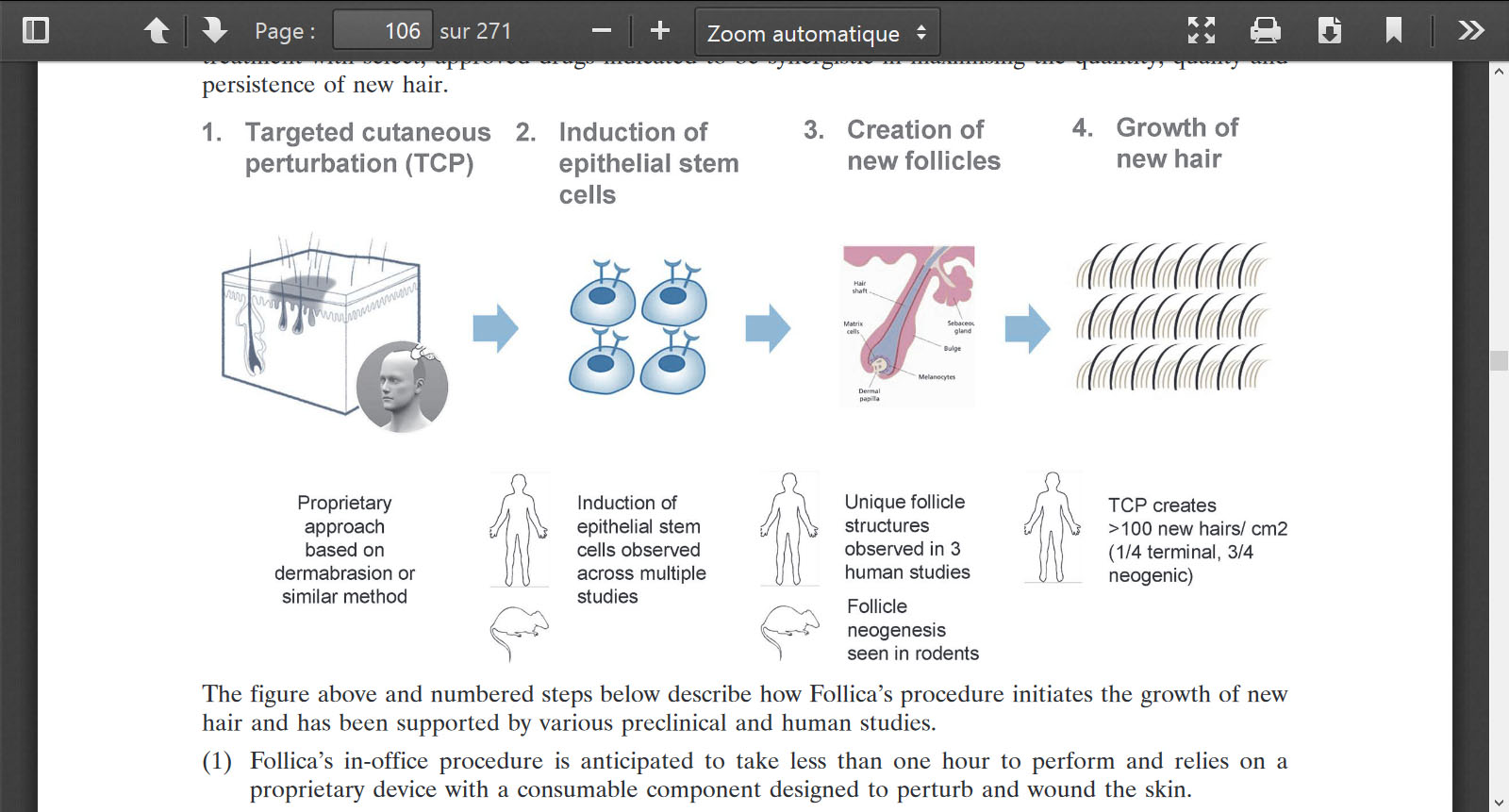
I don't know if you have read this document. On it, you can see few things: (page 106)
http://puretechhealth.com/images/investors/Puretech_Pi_e-Prospectus_Final_T08_CNB-v2.pdf
"Follica is also developing an at-home medical device coupled with a digital service, such as a companion smartphone application for use by patients following the procedure. Follica intends for this device to also be made available to patients experiencing hair loss who are not undergoing the company’s in-office procedure. Market research indicates that the safety profile and improved efficacy of Follica’s product system, in comparison to the use of currently approved drugs alone, could be anticipated to drive sales to those patient populations currently using drug treatments or remaining untreated. It is further anticipated that lower costs and the focus on generation of new hair follicles will potentially capture patients from the transplant procedure population."
" 7.7 Regulatory pathway
The appropriate category of product (medical device, drug or biologic) is determined by the primary mode of action of the product. Follica’s lead product candidate operates primarily by its TCP device. Drugs already in receipt of FDA approval will be used in conjunction with Follica’s TCP device without change to their route of delivery or indication. Therefore, Follica’s lead product candidate is anticipated to be regulated as a medical device Assuming a successful read-out of its next clinical study (i.e. after demonstrating that Follica’s lead product candidate can stimulate cosmetically significant hair growth through TCP and at-home application of an approved drug) Follica expects to seek regulatory approval in the US in late 2017.
7.8 Business plan and commercialisation strategy
Follica is currently developing product specifications for a system consisting of a proprietary medical procedure using medical devices and consumable components. These device concepts are based on the results of the company’s completed human pilot studies and other external supportive research. The company aims to complete product development including the design of high-value disposable devices in 2016 and subsequently initiate a pivotal clinical trial.
Thereafter, Follica plans to seek FDA 510(k) clearance using data from a pivotal clinical trial at multiple clinical sites within and potentially also outside the US. The pivotal trial for the in-office procedure is expected to be initiated during 2016 and would be expected to complete in 2017. If the clinical data is favourable, Follica would plan to seek FDA clearance in 2017, with commercial release to potentially follow in 2018 in the US. The company may consider establishing a relationship with an external corporate partner to assist with the launch and marketing of its products, in the form of a co-promotion or similar agreement. The proceeds allocated to Follica as a result of the Offer are expected to be sufficient to fund Follica through FDA clearance. Follica’s business model focuses on the provision of its patented procedure, devices and consumables to clinicians and their patients seeking treatment for hair loss, as well as the company’s at-home medical devices to healthcare, cosmetics and similar retail organisations, offering hair loss treatments and related devices to their customers. The company intends to develop one or more partnerships to assist in the launch and promotion of its product candidates in both US and other markets, in the form of a co-promotion or similar arrangement. As such, the company’s commercialisation plan involves two
distribution channels:
(1) a professional channel using a direct sales force to target dermatologists, cosmetic surgeons and hair restoration surgeons, as well as other hair loss specialists and clinics who would perform Follica’s patented procedure and resell its devices; and
(2) a direct-to-consumer channel to engage the hair loss patient community, increase awareness of the in-office procedure, as well as drive adoption and use of the at-home device."
http://puretechhealth.com/images/investors/Puretech_Pi_e-Prospectus_Final_T08_CNB-v2.pdf

"Follica is also developing an at-home medical device coupled with a digital service, such as a companion smartphone application for use by patients following the procedure. Follica intends for this device to also be made available to patients experiencing hair loss who are not undergoing the company’s in-office procedure. Market research indicates that the safety profile and improved efficacy of Follica’s product system, in comparison to the use of currently approved drugs alone, could be anticipated to drive sales to those patient populations currently using drug treatments or remaining untreated. It is further anticipated that lower costs and the focus on generation of new hair follicles will potentially capture patients from the transplant procedure population."
" 7.7 Regulatory pathway
The appropriate category of product (medical device, drug or biologic) is determined by the primary mode of action of the product. Follica’s lead product candidate operates primarily by its TCP device. Drugs already in receipt of FDA approval will be used in conjunction with Follica’s TCP device without change to their route of delivery or indication. Therefore, Follica’s lead product candidate is anticipated to be regulated as a medical device Assuming a successful read-out of its next clinical study (i.e. after demonstrating that Follica’s lead product candidate can stimulate cosmetically significant hair growth through TCP and at-home application of an approved drug) Follica expects to seek regulatory approval in the US in late 2017.
7.8 Business plan and commercialisation strategy
Follica is currently developing product specifications for a system consisting of a proprietary medical procedure using medical devices and consumable components. These device concepts are based on the results of the company’s completed human pilot studies and other external supportive research. The company aims to complete product development including the design of high-value disposable devices in 2016 and subsequently initiate a pivotal clinical trial.
Thereafter, Follica plans to seek FDA 510(k) clearance using data from a pivotal clinical trial at multiple clinical sites within and potentially also outside the US. The pivotal trial for the in-office procedure is expected to be initiated during 2016 and would be expected to complete in 2017. If the clinical data is favourable, Follica would plan to seek FDA clearance in 2017, with commercial release to potentially follow in 2018 in the US. The company may consider establishing a relationship with an external corporate partner to assist with the launch and marketing of its products, in the form of a co-promotion or similar agreement. The proceeds allocated to Follica as a result of the Offer are expected to be sufficient to fund Follica through FDA clearance. Follica’s business model focuses on the provision of its patented procedure, devices and consumables to clinicians and their patients seeking treatment for hair loss, as well as the company’s at-home medical devices to healthcare, cosmetics and similar retail organisations, offering hair loss treatments and related devices to their customers. The company intends to develop one or more partnerships to assist in the launch and promotion of its product candidates in both US and other markets, in the form of a co-promotion or similar arrangement. As such, the company’s commercialisation plan involves two
distribution channels:
(1) a professional channel using a direct sales force to target dermatologists, cosmetic surgeons and hair restoration surgeons, as well as other hair loss specialists and clinics who would perform Follica’s patented procedure and resell its devices; and
(2) a direct-to-consumer channel to engage the hair loss patient community, increase awareness of the in-office procedure, as well as drive adoption and use of the at-home device."
Attachments
- Reaction score
- 214
Fantastic discovery! This is pretty encouraging.I don't know if you have read this document. On it, you can see few things: (page 106)
http://puretechhealth.com/images/investors/Puretech_Pi_e-Prospectus_Final_T08_CNB-v2.pdf
"Follica is also developing an at-home medical device coupled with a digital service, such as a companion smartphone application for use by patients following the procedure. Follica intends for this device to also be made available to patients experiencing hair loss who are not undergoing the company’s in-office procedure. Market research indicates that the safety profile and improved efficacy of Follica’s product system, in comparison to the use of currently approved drugs alone, could be anticipated to drive sales to those patient populations currently using drug treatments or remaining untreated. It is further anticipated that lower costs and the focus on generation of new hair follicles will potentially capture patients from the transplant procedure population."
" 7.7 Regulatory pathway
The appropriate category of product (medical device, drug or biologic) is determined by the primary mode of action of the product. Follica’s lead product candidate operates primarily by its TCP device. Drugs already in receipt of FDA approval will be used in conjunction with Follica’s TCP device without change to their route of delivery or indication. Therefore, Follica’s lead product candidate is anticipated to be regulated as a medical device Assuming a successful read-out of its next clinical study (i.e. after demonstrating that Follica’s lead product candidate can stimulate cosmetically significant hair growth through TCP and at-home application of an approved drug) Follica expects to seek regulatory approval in the US in late 2017.
7.8 Business plan and commercialisation strategy
Follica is currently developing product specifications for a system consisting of a proprietary medical procedure using medical devices and consumable components. These device concepts are based on the results of the company’s completed human pilot studies and other external supportive research. The company aims to complete product development including the design of high-value disposable devices in 2016 and subsequently initiate a pivotal clinical trial.
Thereafter, Follica plans to seek FDA 510(k) clearance using data from a pivotal clinical trial at multiple clinical sites within and potentially also outside the US. The pivotal trial for the in-office procedure is expected to be initiated during 2016 and would be expected to complete in 2017. If the clinical data is favourable, Follica would plan to seek FDA clearance in 2017, with commercial release to potentially follow in 2018 in the US. The company may consider establishing a relationship with an external corporate partner to assist with the launch and marketing of its products, in the form of a co-promotion or similar agreement. The proceeds allocated to Follica as a result of the Offer are expected to be sufficient to fund Follica through FDA clearance. Follica’s business model focuses on the provision of its patented procedure, devices and consumables to clinicians and their patients seeking treatment for hair loss, as well as the company’s at-home medical devices to healthcare, cosmetics and similar retail organisations, offering hair loss treatments and related devices to their customers. The company intends to develop one or more partnerships to assist in the launch and promotion of its product candidates in both US and other markets, in the form of a co-promotion or similar arrangement. As such, the company’s commercialisation plan involves two
distribution channels:
(1) a professional channel using a direct sales force to target dermatologists, cosmetic surgeons and hair restoration surgeons, as well as other hair loss specialists and clinics who would perform Follica’s patented procedure and resell its devices; and
(2) a direct-to-consumer channel to engage the hair loss patient community, increase awareness of the in-office procedure, as well as drive adoption and use of the at-home device."
Dragonborn
New Member
- Reaction score
- 1
The most interesting thing about this is the neogenesis mentioned and the figure of 100 hairs per cm^2. If this is true they have a money maker here.
THey refer to:
Honestly if they proceed with this and this delivers on the 100 new hairs per square centimeter in term of results than this would be a game changer.
I guess we'll know more in 2017.
THey refer to:
. I'm guessing either minoxidil or PDG2 antagonist.Drugs already in receipt of FDA approval will be used in conjunction with Follica’s TCP device without change to their route of delivery or indication
Honestly if they proceed with this and this delivers on the 100 new hairs per square centimeter in term of results than this would be a game changer.
I guess we'll know more in 2017.
- Reaction score
- 214
100 hairs per cm^2 is only half of the average density of a caucasian. Do you think the proceedure could be repeated for more coverage?The most interesting thing about this is the neogenesis mentioned and the figure of 100 hairs per cm^2. If this is true they have a money maker here.
THey refer to:
. I'm guessing either minoxidil or PDG2 antagonist.
Honestly if they proceed with this and this delivers on the 100 new hairs per square centimeter in term of results than this would be a game changer.
I guess we'll know more in 2017.
- - - Updated - - -
And only 25 of them are terminal.100 hairs per cm^2 is only half of the average density of a caucasian. Do you think the proceedure could be repeated for more coverage?
- - - Updated - - -
And WTH is a neogenic hair? What's the difference between neogenic and terminal?100 hairs per cm^2 is only half of the average density of a caucasian. Do you think the proceedure could be repeated for more coverage?
- - - Updated - - -
And only 25 of them are terminal.
- Reaction score
- 2,634
100 hairs per cm^2 is only half of the average density of a caucasian. Do you think the proceedure could be repeated for more coverage?
- - - Updated - - -
And only 25 of them are terminal.
- - - Updated - - -
And WTH is a neogenic hair? What's the difference between neogenic and terminal?
Terminal = thick
Neogenic = new hair (could be vellus or terminal)
- Reaction score
- 214
Wow, so this procedure has been shown to grow only 1/8 of the average density of hair on the scalp.
- Reaction score
- 2,634
Wow, so this procedure has been shown to grow only 1/8 of the average density of hair on the scalp.
Just keep doing the procedure until you've got enough hair and you're cured.
- Reaction score
- 214
Yeah but only if you can do it on an area that already has hair. Do you think that will be an option?Just keep doing the procedure until you've got enough hair and you're cured.

