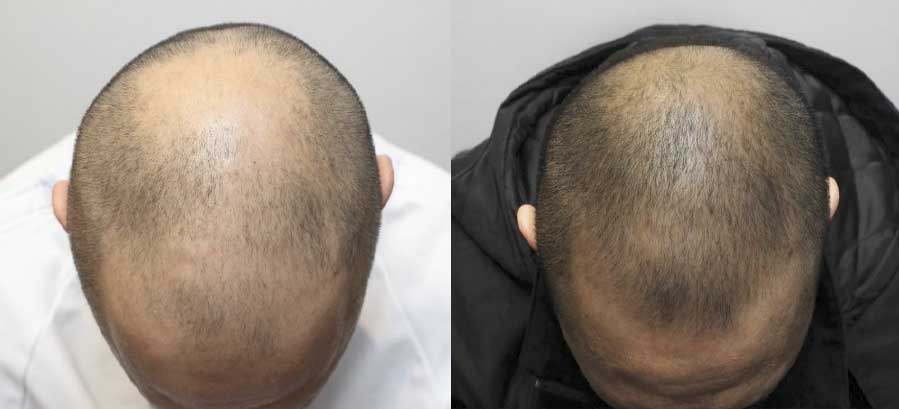
SAN DIEGO, July 28, 2015 /PRNewswire/ -- Kerastem Technologies announce that the Company has received notice of conditional approval from the U.S. FDA Center For Biologics Evaluation and Research (CBER) Office of Cellular, Tissue and Gene Therapies to conduct a clinical trial investigating the safety and feasibility of the Company's technology for the treatment of female and early male pattern baldness (androgenic alopecia).
The phase II study, known as the STYLE trial, follows initial clinical work in Europe and Japan. This clinical experience served as a basis for the FDA IDE submission. The data will be presented in September at the 2015 International Society of Hair Restoration Surgery meeting in Chicago. STYLE is a 70 patient controlled trial that is expected to begin enrollment in late 2015 at up to eight (8) centers within the United States. The primary endpoint is safety and tolerability of the treatment and secondary endpoints include change in hair growth and density.
According to Principal Investigator Ken Washenik, MD, PhD, Medical Director--Bosley Medical Group, and Clinical Assistant Professor, Department of Dermatology, New York University School of Medicine, "I have been heavily involved in the care and research of alopecia patients and treatments for over 20 years, including the use of cellular based approaches. As principal investigator of the STYLE trial, I look forward to evaluating this exciting new approach to the treatment of hair loss."
The global hair loss market is a $7B cash pay business growing annually at 4.8%. "The hair market has few acceptable solutions for early hair loss, particularly in women," according to Bradford Conlan, CEO of Kerastem. "STYLE represents a defined clinical pathway in the United States and complements our commercial efforts in Europe and Asia," added Mr. Conlan. The Company's platform technology is CE Mark approved and available for patients with alopecia in Europe. Kerastem is currently engaged in market development with select commercial partners.
For more information on STYLE, visit www.kerastem.com
About Kerastem
Kerastem Technologies is a private company owned by Bimini Technologies, and holds global and exclusive rights to commercialize Cytori Therapeutics, Inc.'s Celution® Technology for alopecia and hair related indications. The Bimini portfolio of products also includes Puregraft (www.puregraft.com, www.thefatexperts.com) the world's leading fat grafting technology.
The STYLE trial is now listed on www.clinicaltrials.gov at the following location:
https://clinicaltrials.gov/ct2/show/NCT02503852?term=Kerastem&rank=1
Contact: Support@kerastem.com
Logo - http://photos.prnewswire.com/prnh/20150728/247048LOGO
http://www.prnewswire.com/news-rele...al-from-fda-for-alopecia-trial-300119592.html
...........
The phase II study, known as the STYLE trial, follows initial clinical work in Europe and Japan. This clinical experience served as a basis for the FDA IDE submission. The data will be presented in September at the 2015 International Society of Hair Restoration Surgery meeting in Chicago. STYLE is a 70 patient controlled trial that is expected to begin enrollment in late 2015 at up to eight (8) centers within the United States. The primary endpoint is safety and tolerability of the treatment and secondary endpoints include change in hair growth and density.
According to Principal Investigator Ken Washenik, MD, PhD, Medical Director--Bosley Medical Group, and Clinical Assistant Professor, Department of Dermatology, New York University School of Medicine, "I have been heavily involved in the care and research of alopecia patients and treatments for over 20 years, including the use of cellular based approaches. As principal investigator of the STYLE trial, I look forward to evaluating this exciting new approach to the treatment of hair loss."
The global hair loss market is a $7B cash pay business growing annually at 4.8%. "The hair market has few acceptable solutions for early hair loss, particularly in women," according to Bradford Conlan, CEO of Kerastem. "STYLE represents a defined clinical pathway in the United States and complements our commercial efforts in Europe and Asia," added Mr. Conlan. The Company's platform technology is CE Mark approved and available for patients with alopecia in Europe. Kerastem is currently engaged in market development with select commercial partners.
For more information on STYLE, visit www.kerastem.com
About Kerastem
Kerastem Technologies is a private company owned by Bimini Technologies, and holds global and exclusive rights to commercialize Cytori Therapeutics, Inc.'s Celution® Technology for alopecia and hair related indications. The Bimini portfolio of products also includes Puregraft (www.puregraft.com, www.thefatexperts.com) the world's leading fat grafting technology.
The STYLE trial is now listed on www.clinicaltrials.gov at the following location:
https://clinicaltrials.gov/ct2/show/NCT02503852?term=Kerastem&rank=1
Contact: Support@kerastem.com
Logo - http://photos.prnewswire.com/prnh/20150728/247048LOGO
http://www.prnewswire.com/news-rele...al-from-fda-for-alopecia-trial-300119592.html
...........