Edit: This comes up over and over again so I'm pinning it to the top. These are quotes from two studies explaining why your prolactin levels have nothing to do with hair loss.
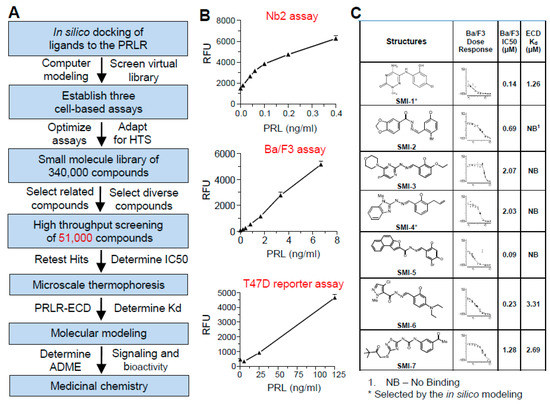
HMI-115 is a monoclonal antibody for the PRLR(prolactin receptor). It was licensed by Bayer to Hopemed Inc. It is currently in clinical trials for endometriosis and androgenetic alopecia. In early 2022 the company received FDA approval to conduct a phase II trial in the US for Androgenetic Alopecia, and soon after they received approval to begin a 6 month phase I trial in Australia that should demonstrate efficacy. The Australian trial is currently recruiting. The company has said that they hope to have it on the market by 2024, but that will be a difficult target to meet. They originally planned to move straight into a phase II trial for Androgenetic Alopecia since they had already completed a phase I for endometriosis. The FDA approved that, but Australia did not. The phase II trial now appears to be on hold until the Phase I in Australia is completed. They are currently conducting a phase II in endometriosis.
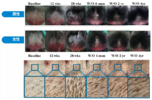
Most of the information we have about the treatment comes from this patent. The patent details a trial conducted in stumptailed macaques. This is the best available model for human male pattern baldness. Stumptailed macaques have androgenetic alopecia like humans. They go bald when they hit puberty, and finasteride prevents this. All treatments that have been tested on both humans and macaques have similar efficacy in both, so that's a very good sign that this drug will work in humans too. What makes this treatment so promising is the amount of hair that the macaques regrew in this trial, and the quality of that hair. No other treatment has come close to growing this much hair in stumptailed macaques. Not even the combination of finasteride and minoxidil could come close. Additionally, the results last longer than other treatments. The macaques were injected subcutaneously with the antibody once every two weeks for 28 weeks, after which they had nearly full regrowth. Even after 4 years without treatment the macaques were still well above baseline. Other trials measure hair weight or the number of vellus hairs regrown. These measurements on the graphs below represent "thick terminal hairs", and you can see by the trichoscope image that miniaturized hairs returned to full thickness. Additionally, results did not plateau during the treatment period, so if treatment were continued for longer than 6 months they very likely would have had even more regrowth.


From the patent:
How did we get to this point? The antibody was developed by Bioinvent, and licensed to Bayer in 2008. Some years later Bayer finally got around to developing the drug for endometriosis. They conducted a phase I trial that was published in 2018. During the preclinical work for this trial it was noticed that the drug increased hair growth in mice. Bayer, however, was not interested in pursuing a drug for male pattern baldness. They had begun an initiative to focus on women's health issues. Fortunately for us Rui-Ping Xiao was already working with Bayer on another project as head of the IMM primate center in Beijing. She asked Bayer if she could test the drug on bald, stumptailed macaques, and they agreed. When the results came in she formed Hopemed Inc., and purchased the global rights to the drug from Bayer in 2019.
I will post some links to interviews. Unfortunately some are no longer available or have been revised.


 www.prnewswire.com
https://enmobile.prnasia.com/releas...apabilities-of-global-innovation-317537.shtml
www.prnewswire.com
https://enmobile.prnasia.com/releas...apabilities-of-global-innovation-317537.shtml
dopamine, known as an inhibitor of PRL pituitary secretions, has no effect on PRL or PRL-R expression in human HFs
We also show that the catagen-promoting activity of PRL is independent of the hypothalamus-pituitary-adrenal axis and systemic hormone levels. It applies to HFs of a mammalian species with mosaic and seasonally independent HF cycling
HMI-115 is a monoclonal antibody for the PRLR(prolactin receptor). It was licensed by Bayer to Hopemed Inc. It is currently in clinical trials for endometriosis and androgenetic alopecia. In early 2022 the company received FDA approval to conduct a phase II trial in the US for Androgenetic Alopecia, and soon after they received approval to begin a 6 month phase I trial in Australia that should demonstrate efficacy. The Australian trial is currently recruiting. The company has said that they hope to have it on the market by 2024, but that will be a difficult target to meet. They originally planned to move straight into a phase II trial for Androgenetic Alopecia since they had already completed a phase I for endometriosis. The FDA approved that, but Australia did not. The phase II trial now appears to be on hold until the Phase I in Australia is completed. They are currently conducting a phase II in endometriosis.
Most of the information we have about the treatment comes from this patent. The patent details a trial conducted in stumptailed macaques. This is the best available model for human male pattern baldness. Stumptailed macaques have androgenetic alopecia like humans. They go bald when they hit puberty, and finasteride prevents this. All treatments that have been tested on both humans and macaques have similar efficacy in both, so that's a very good sign that this drug will work in humans too. What makes this treatment so promising is the amount of hair that the macaques regrew in this trial, and the quality of that hair. No other treatment has come close to growing this much hair in stumptailed macaques. Not even the combination of finasteride and minoxidil could come close. Additionally, the results last longer than other treatments. The macaques were injected subcutaneously with the antibody once every two weeks for 28 weeks, after which they had nearly full regrowth. Even after 4 years without treatment the macaques were still well above baseline. Other trials measure hair weight or the number of vellus hairs regrown. These measurements on the graphs below represent "thick terminal hairs", and you can see by the trichoscope image that miniaturized hairs returned to full thickness. Additionally, results did not plateau during the treatment period, so if treatment were continued for longer than 6 months they very likely would have had even more regrowth.



From the patent:
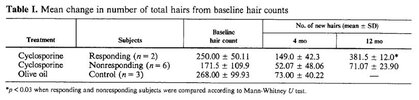
Based on hair diameters, the number of vellus and terminal hairs per cm2 was determined. Both measurements (hair diameters and count) were performed using Datinf TrichoScan Smart Software in semi-automated manner with manual curating. For consistency reasons each region was analyzed by the same observer over time. The obtained data showed an increased terminal hair count in the previously bald region in 9 out of 11 monkeys. The increase in hair count ranged from 50 - 220 hairs/cm2 in responder monkeys (see figure 2B). The effect was observed in male and female monkeys. Younger animals responded better than seniles ones. No plateau of efficacy was reached in 6 months of treatment with the antibody mat3. Best effects with regard to increase of absolute numbers of terminal hairs as well as with regard to percentage increase was observed in previously bald areas, i.e. such areas where vellus hairs were dominating before treatment. Such areas had initially been considered the most difficult to treat since baldness of these areas was preexisting for decades in some of the monkeys. Six months after ceasing the treatment, no further increase of hair diameters or increase of terminal hairs has been observed. However, there was also only a marginal (insignificant) drop from the level reached at the end of the study. The proportion of terminal hairs was significantly higher than before start of treatment 12 months ago, i.e. a long lasting effect of treatment was observed.
25 years of age for a macaque is equivalent to ~66 years of age in a human. The drug worked on three of these animals, and on all 6 of the younger ones.5 out of 11 monkeys were >25 years of age and were considered particularly difficult to treat. However, three of those responded to compound treatment. Notably, completely bald scalp areas responded best. The PRLR antibody mat3 showed more efficacy and was effective following a more convenient regime (s.c. twice per month) than the only two approved medications for M/FPHL (minoxidil (topical, daily), and finasteride (oral, once daily, men only). (See example 4 and figures 2-3)).
There were no apparent safety or tolerability signals during or after 6 month treatment.
It could be demonstrated that there was a robust and visible efficacy in bald and transition areas.
How did we get to this point? The antibody was developed by Bioinvent, and licensed to Bayer in 2008. Some years later Bayer finally got around to developing the drug for endometriosis. They conducted a phase I trial that was published in 2018. During the preclinical work for this trial it was noticed that the drug increased hair growth in mice. Bayer, however, was not interested in pursuing a drug for male pattern baldness. They had begun an initiative to focus on women's health issues. Fortunately for us Rui-Ping Xiao was already working with Bayer on another project as head of the IMM primate center in Beijing. She asked Bayer if she could test the drug on bald, stumptailed macaques, and they agreed. When the results came in she formed Hopemed Inc., and purchased the global rights to the drug from Bayer in 2019.
I will post some links to interviews. Unfortunately some are no longer available or have been revised.


Hope Medicine announced global license agreement with Bayer AG to advance the development and commercialization of the monoclonal antibody directed against prolactin (PRL) receptor
/PRNewswire/ -- Hope Medicine Inc. announced today that they have signed a world-wide exclusive license agreement with Bayer AG on the development and...
https://transkript.de/news/haariges-geschaeft.htmlThe team behind the financially strong start-up Hope Medicine was so impressed by the preclinical data that they submitted an offer to Bayer. This was apparently so good that it successfully outpaced other interested parties and the option to pursue the program internally at Bayer. As it became known in April, the German pharmaceutical company will receive an upfront payment from Hope Medicine as well as further payments upon reaching certain development stages and a share of sales.
“The surprising hair growth caused by treatment with the active ingredient was observed in preclinical experiments with mice: in animals with artificially increased prolactin levels, shaved areas grew closed again within two weeks.”
On a schedule, HMI-115, which treats lymphatic fibroids, is first expected to be approved for sale in 2024 and its Core Pipeline when asked about the impact of the new crown outbreak, Xiao Ruiping admitted that its research process is still on schedule
Last edited: