You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
f*** Allergan. Serial Dropping Of Future Treatments.
- Thread starter jamesbooker1975
- Start date
Faster, not thicker. Great difference.Well on biotin my hairs were growing 2 even 3 times faster...
Fevi is going to be for asthma, not for Hair Loss, just like Dutasteride is for prostate not for hair, but is off lable use anyway. I was talking about Seti anyway...
I’m just saying the seti claims are very anecdotal. I heard so many claims of it not working even on high doses. I don’t have faith in either as i don’t think it’s the right path
Of course it is difference. However it still have impact(biotin) for hair.
Well, we will see. I am finding this drug as my last hope for hair loss, If it won't work, i will probably give up.
Btw, where did you heard from people that seti did not work for them??
Well, we will see. I am finding this drug as my last hope for hair loss, If it won't work, i will probably give up.
Btw, where did you heard from people that seti did not work for them??
Last edited:
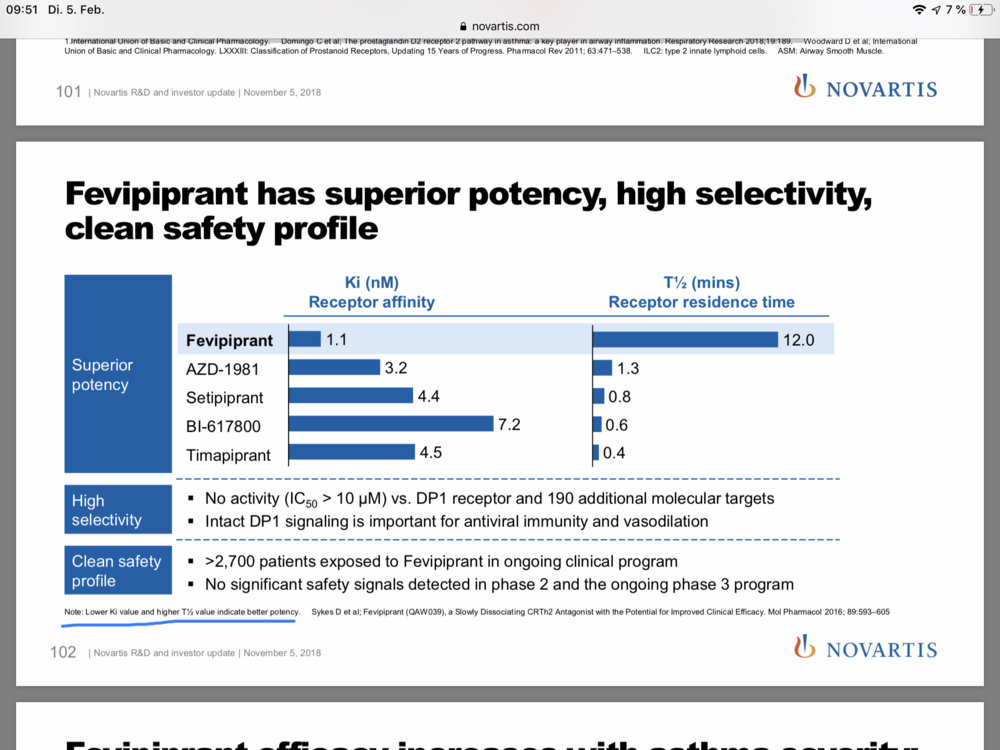
This is from November, but this also shows how much superior Fevipiprant is compared to Setipiprant, probably even if Setipiprant is used at a dose 4 times as high as Fevipiprant. And 2g of Setipiprant seems to be the minimal dose to have some positive effect.
https://www.novartis.com/sites/www.novartis.com/files/2018-11-05-r-d-day-investor-presentation.pdf
Unfortunately, Novartis has still no plans to do a PoC trial for hair loss. (Maybe they are also waiting for Allergan‘s trial result.) Others, however, also seem to have plans to develop a oral dp2/crth2 receptor antagonist against hair loss. They completed a phase 1 trial a year ago and next stages are „under consideration“. ( https://www.folliclethought.com/new-company-brickell-biotech/ ) For me it seems encouraging that also other companies believe dp2 blockers could work, but I also think it‘s a bit strange for them to develop it considering Fevipiprant and Setipiprant are both at later stages of development.
https://www.novartis.com/sites/www.novartis.com/files/2018-11-05-r-d-day-investor-presentation.pdf
Unfortunately, Novartis has still no plans to do a PoC trial for hair loss. (Maybe they are also waiting for Allergan‘s trial result.) Others, however, also seem to have plans to develop a oral dp2/crth2 receptor antagonist against hair loss. They completed a phase 1 trial a year ago and next stages are „under consideration“. ( https://www.folliclethought.com/new-company-brickell-biotech/ ) For me it seems encouraging that also other companies believe dp2 blockers could work, but I also think it‘s a bit strange for them to develop it considering Fevipiprant and Setipiprant are both at later stages of development.
- Reaction score
- 317
There is https://brickellbio.com/rd/pipeline/#/bbi5000.html
Plus there is pulmagen and some other potent crth2 inhibitors like ACT. It is not rlly suprising they drop that inefficient crap as they wont get seti out earlier than fevi.
Cots stated just in august that they checked females for increased pgd2 levels, which seemed to be the case. I wouldnt be surprised if seti either goes on or not. Either way, this has less to do with the fact if it works or not.
Cots got also an interesting paper regarding Shh in the pipeline.
Plus there is pulmagen and some other potent crth2 inhibitors like ACT. It is not rlly suprising they drop that inefficient crap as they wont get seti out earlier than fevi.
Cots stated just in august that they checked females for increased pgd2 levels, which seemed to be the case. I wouldnt be surprised if seti either goes on or not. Either way, this has less to do with the fact if it works or not.
Cots got also an interesting paper regarding Shh in the pipeline.
So, it seems like Allergan submitted their results on clinicaltrials.gov.
https://clinicaltrials.gov/ct2/show/results/NCT02781311
However, they are not yet visible for the public as it is pending Quality Control Review by the National Library of Medicine. But for me it only seems like a question of time until they become publicly available.
https://clinicaltrials.gov/ct2/show/results/NCT02781311
However, they are not yet visible for the public as it is pending Quality Control Review by the National Library of Medicine. But for me it only seems like a question of time until they become publicly available.
To the people on here that are educated in the field, what is your estimate on when these results will be available to the public?...why so secretive? Why cant they just tell us the results at this point? Haven't we waited long enough? SmhSo, it seems like Allergan submitted their results on clinicaltrials.gov.
https://clinicaltrials.gov/ct2/show/results/NCT02781311
However, they are not yet visible for the public as it is pending Quality Control Review by the National Library of Medicine. But for me it only seems like a question of time until they become publicly available.
I think the fact that they submitted means there is a high probability of success. As you can see, the VAST majority of studies don't even produce results:
https://clinicaltrials.gov/ct2/resu...term=&cntry=&state=&city=&dist=&Search=Search
They did, so maybe that means they're good. If they got no results, they would have NO profitable incentive to formalize it and publish.
Looking at other studies, it'll be about 2 months before being posted.
https://clinicaltrials.gov/ct2/resu...term=&cntry=&state=&city=&dist=&Search=Search
They did, so maybe that means they're good. If they got no results, they would have NO profitable incentive to formalize it and publish.
Looking at other studies, it'll be about 2 months before being posted.
Ok nice...so when is fevi due out?I think the fact that they submitted means there is a high probability of success. As you can see, the VAST majority of studies don't even produce results:
https://clinicaltrials.gov/ct2/resu...term=&cntry=&state=&city=&dist=&Search=Search
They did, so maybe that means they're good. If they got no results, they would have NO profitable incentive to formalize it and publish.
Looking at other studies, it'll be about 2 months before being posted.
Do you know if Novartis still has 2020 as the date of release for fevi?
Not sure about that. On their last presentations they said they would do the FDA/EMA filing in 2020. But maybe they go for a fast track approval, as asthma is a serious condition (compared to hairloss) and Novartis has enough money to pay for that. That would mean it could be out in late 2020 or early 2021 if everything goes right. But they seem quite confident about Fevi to be a successful drug (for asthma at least
