- Reaction score
- 1,332
Ok guys thought it would be a good idea to give an update about androgenetic alopecia. Basically I just want to give an insight about what the latest research is showing, but also define some basic characteristics of androgenetic alopecia (perhaps you might know allot of this) We’ll start from the beginning;
In the 50’s experiments were done by Dr. James Hamilton. He was also the first one who created the term “androgenetic alopecia”. He saw that men who had been castrated before puberty never went bald. Interestingly when he injected testosterone into these castrated men, some suddenly started balding but some did not. Most notably the ones with a family history of baldness started balding.
Why were his experiments so important? Well he showed that baldness is androgen dependent, because castrated men before puberty showed no signs of balding. He also showed that there is a genetic side to the story because when he injected some with testosterone, not everyone started balding. This is also why we call it “androgenetic” alopecia. Because for baldness to occur as we know it you need both androgens + genetics.
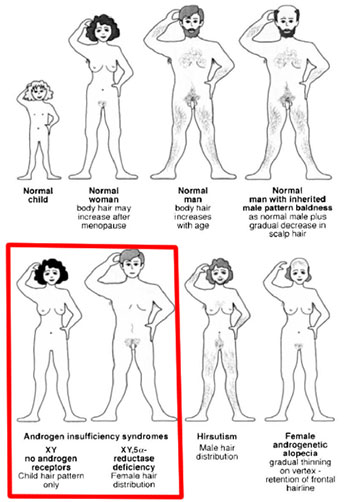
To verify this even more Imperato- McGinley studied pseudo-hermaphrodites in the Dominican republic. These pseudo-hermaphrodites were born looking like a girl with female genitals. At puberty however they virilized, developing musculature, a deeper voice and would even develop a penis some of the time. They found out that these pseudo-hermaphrodites had a deficiency of type-2 5-reductase. Then you have people who suffer from androgen insensitivity syndrome. This condition results in a partial or complete inability of cells to respond to androgens. Guess what? Well both of these type people who suffer from this condition never display androgenetic alopecia. This is again a very important observation to make that androgenetic alopecia is androgen dependent. A fun fact is that Merck developed a drug based on the study of Imperato- McGinley; finasteride a type-2 5-reductase inhibitor (5ar2) as we all know it.
Next I want to explain hair follicle cycling in short. A hair follicle cycles as you may know. Everyone experiences this, even your norwood 1 friend. This primarily exists of the following stages; anagen, catagen and lastly telogen, this cycle repeats itself. Anagen is the active growth phase of the hair, catagen is the regression phase and telogen is the resting phase. Now look at the following picture;
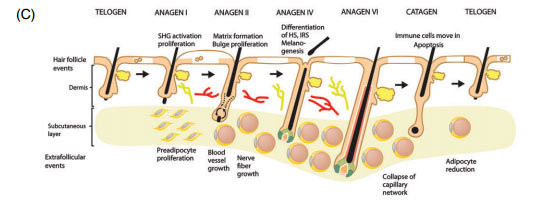
What important to know about these cycles is that the hair follicle itself, specifically the micro environment of the hair follicle is able to communicate with the macro environment (surrounding tissue) . For instance in telogen a hair will lack vascularization and blood vessels, not only that it will also lack adipose tissue. In anagen however there is a tremendous increase in vascularization and also adipose tissue. This leads to a thicker dermis in anagen, and a thinner dermis in telogen. Scalp hair follicles reside generally 80-90% in anagen, 1-2% in catagen and 10-15% in telogen. Ok this is enough for general cycling.
Back to androgenetic alopecia again. You should already know that androgenetic alopecia is androgen dependent and has a genetic basis. However for androgenetic alopecia to occur these androgens need to bind to the androgen receptor in the hair follicle. Because as you know people with androgen insensitivity syndrome have hormones but they have impaired androgen receptors and they don’t bald. So where are the androgen receptors located in the hair follicle? They are solely located in the dermal papilla at the base of the hair follicle;

So in order for androgenetic alopecia to occur, androgens which are expressed in the blood need to bind to the androgen receptors located in the dermal papilla. A treatment for example like RU-58841 or CB-03 binds to the androgen receptors there and will stop androgens to attach to these androgen receptors, preventing or slowing androgenetic alopecia. There is evidence that people suffering from androgenetic alopecia have increased androgen receptor expression and increased 5ar2 expression.
In androgenetic alopecia as you guys may very well know too is that with each cycle the hair becomes more miniaturized. The hair becomes thinner, shallower and keeps reducing each cycle until you are basically left with almost nothing. Important to know is that the dermal papilla cells specify hair size, shape and the reduction of them causes a follicular decline (1), (2).
_________________________________________________________________________________________________________________________________________________________________________________________________________________________
Now to the (latest) advancements about the pathology of androgenetic alopecia;
In the study named “Premature Senescence of Balding Dermal Papilla Cells In Vitro Is Associated with p16INK4a Expression”(3). They confirmed as other studies already did(4) that balding dermal papilla cells grow way slower in vitro when compared to non-balding dermal papilla cells. Not only that they found out that balding dermal papilla cells undergo premature senescence. This was associated with the following in the balding dermal papilla cells;
- Increased Expression of p16ink4a
- Increased Expression of pRb
- Expression of senescence-associated β-galactosidase
- Loss of expression of bmi-1
- Increased expression of oxidative stress and dna damage markers like HSP-27, ATM and ATR
Now a study published in November 2014 called “ Androgen Receptor Accelerates Premature Senescence of Human Dermal Papilla Cells in Association with DNA Damage” (5) found ;
- Also a increase of p16ink4a in balding dermal papilla cells and not in non-balding in vitro
- DNA damage accompanied with senescence in the balding dermal papilla cells
- Most importantly when you remove the androgen receptor from the balding dermal papilla cells DNA damage does NOT occur and senescence does NOT occur.
- The dermal papilla cells changed in morphology, they enlarged.
A other study in 2009 called “Proliferation, DNA repair and apoptosis in androgenetic alopecia (6)” found out that in comparison with occipital scalp and frontal balding scalp;
- P53 was overexpressed frontal balding scalp
- P53 showed a inverse correlation with APE-1 (dna damage repair marker)
Another study called "Analysis of microRNA expression profile in 5α -dihydrotestosterone-treated normal human dermal papilla cells" (http://www.papersearch.net/view/detail.asp?detail_key=27731029)
[video=youtube;0klARAxOYiI]https://www.youtube.com/watch?v=0klARAxOYiI[/video]
_____________________________________________________________________________________________________________________________________________________________________________________________________________________________
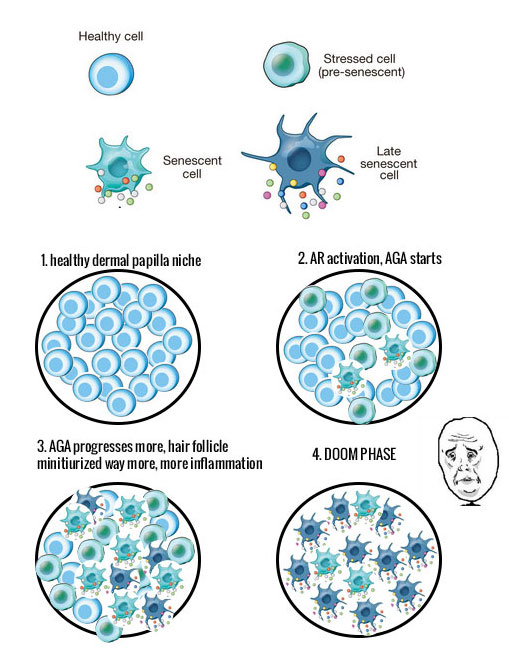
Short explanation about senescence in general
Read the following picture this is taken from “Cellular senescence: when bad things happen to good cells(7)”. It is NON-Androgenetic Alopecia related but related to general cellular senescence;
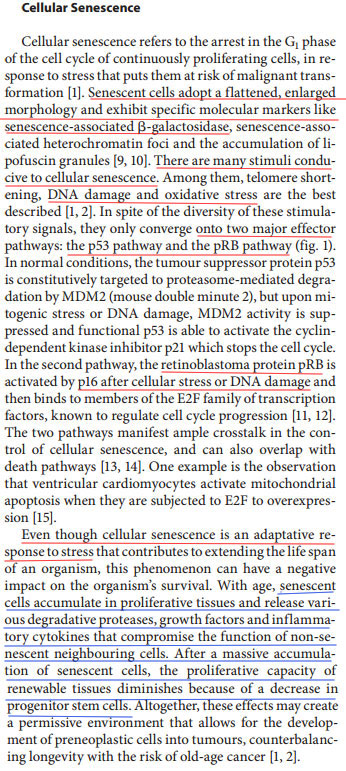
I underlined the parts in red that compare with the evidence from the androgenetic alopecia studies. P16(ink4a), pRB, P53 etc. In blue I underlined something that is very typical and extremely important for cellular senescence and that is that these cells release pro-inflammatory factors and even compromise the function of non-senescent healthy cells. Not only that note how they say that it may even lead to a decrease or even a loss in progenitor stem cells. Well as you guys know we are coping with inflammation too in androgenetic alopecia, there are a **** ton of inflammatory factors expressed.
Not only that we have a lack of CD34+ and CD-200 progenitors (http://www.ncbi.nlm.nih.gov/pubmed/21206086)
Even the whole morphology of the hair follicle changes.....
1. http://www.ncbi.nlm.nih.gov/pubmed/23487317
2. http://www.nature.com/jid/journal/v1.../5600534a.html
3. http://www.nature.com/jid/journal/v1.../5701147a.html
4. http://www.ncbi.nlm.nih.gov/pubmed/8...ct&holding=npg
5. http://www.plosone.org/article/info%...l.pone.0079434
6. http://www.ncbi.nlm.nih.gov/pubmed/18702626
7. http://www.nature.com/nrm/journal/v8...l/nrm2233.html
______________________________________________________________________________________________________________________________________________________________________________________________________________________________
Further explanation of senescence (why does it happen in general?)
Now for the next part. What trigger can make cells go into a senescent state? What trigger can activate these pathways? Well this can be DNA damage (telomere shortening, single- and double-strand breaks), oncogenic mutations, reactive metabolites (ROS, ceramides, fatty acids, high glucose), increased mTOR activity and proteotoxic stress. As you see in the following picture (This picture most likely doesn't reflect the exact pathway in Androgenetic Alopecia which cause cellular senescence obviously but should give a global view);
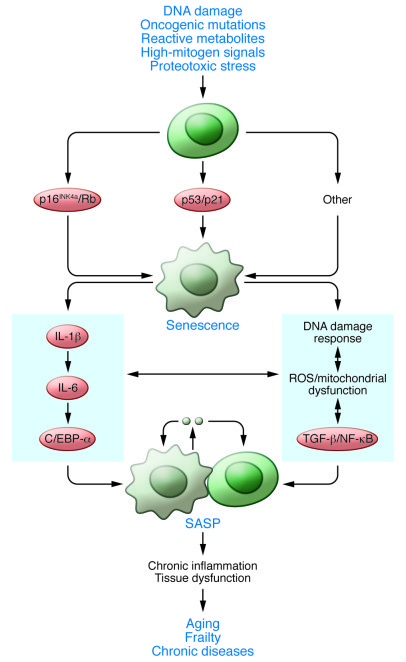
Senescence-associated secretory phenotype (SASP) as you see here in the picture above is the result of senescent cells. Senescent cells secrete pro-inflammatory factors. I quote;
“Recent evidence in fibroblasts and epithelial cells has shown that cellular senescence is accompanied by a striking increase in the secretion of 40–80 factors that participate in intercellular signaling. Secretion of this set of factors has been termed the “senescence-associated secretory phenotype”, or SASP. (Inflammatory Networks during Cellular Senescence: Causes and Consequences (4)).
_______________________________________________________________________________________________________________________________________________________________________________________________________________
What factors could be driving the senescence in Androgenetic Alopecia though?
A interesting fact is that androgens act as DNA-damaging agents that generate DNA double-strained breaks (DSBs) which may contribute to the senescence of dermal papilla cells. A other study shows that cultured balding dermal papilla cells secrete more TGF-β (1) and this factor has been shown to stimulate oxidative stress (ROS) (2). Interestingly in normal prostate epithelial cells the androgen receptor has been shown to drive cellular senescence too but without expression of DNA damage or p16ink4a(3). So this reflects the diversity of biological responses the AR can have in relation to senescence in different cell types.
1. http://www.ncbi.nlm.nih.gov/pubmed/1...ct&holding=npg
2. http://www.ncbi.nlm.nih.gov/pubmed/8...ct&holding=npg
3. http://www.ncbi.nlm.nih.gov/pmc/arti...?report=reader
4. http://www.ncbi.nlm.nih.gov/pmc/arti...8/#!po=15.0000
_____________________________________________________________________________________________________________________________________________________________________________________
Trying to propose a link to what causes senescence in Androgenetic Alopecia. Using an Androgenetic Alopecia genetic profile study (don't read to much hypothetical talk);
Using this study; http://www.ncbi.nlm.nih.gov/pmc/articles/PMC4164265/
This is the table with differentially expressed genes in androgenetic alopecia with changes of -2 or +2 , basically the ones that stand out the most; http://www.ncbi.nlm.nih.gov/pmc/arti...ort=objectonly
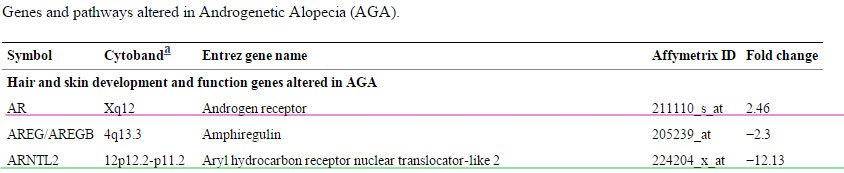
Obviously as said there is evidence again that the androgen receptor is fcked in Androgenetic Alopecia, they found a 2.46 fold increase of this compared to control as you can see in the link just above and in the picture here above where I underlined it in purple. I underlined the Aryl hydrocarbon receptor nuclear translocator-like 2 because that one is the most differentially expressed in this research paper with a fold change of -12.13. This gene encodes a protein that is a co-factor in transcriptional regulation by hypoxia-inducible factor 1 (HIF-1A). We'll come back to this later.
Now they say that the most significant pathway altered in androgenetic alopecia is NOTCH signalling which consists of 29 genes. Furthermore they refer to a other study which found a suspectibility locus for Androgenetic Alopecia at chromosome 20p, and JAGGED1 (JAG1) gene is a ligand for the NOTCH receptor and maps to this. They propose that activation of the androgen receptor leads to loss of NOTCH signalling which results in miniaturization;
Nonetheless, let's dig a bit further and connect the dots a bit further ourself from the information in this study. We have;
Let's propose a hypothesis and try to connect those 3 together. First we are going to look at if NOTCH and the androgen receptor have interactions with each other as proposed;
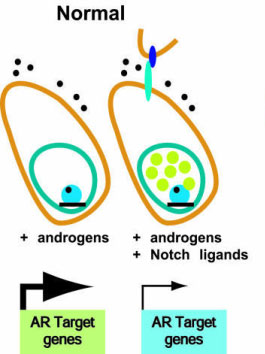
NOTCH is also expressed abundantly in hair follicles. I underlined here evidence that the activation of AR upon androgen binding can for instance downregulate NOTCH signalling and the ligand JAGGED1 which maps to a suspectible locus in Androgenetic Alopecia. I'm not going to go to deep into this, but there are many studies which show androgen receptor interaction with NOTCH, you can look them up yourself if you want.
So yes perhaps in Androgenetic Alopecia because of an overexpressed androgen receptor there is loss of NOTCH signalling. Now we go to the second point , can NOTCH signalling be connected to the Altered Aryl hydrocarbon receptor nuclear translocator-like 2 with a -12.13 fold gene expression in Androgenetic Alopecia? As I this gene encodes a protein that is a co-factor in transcriptional regulation by hypoxia-inducible factor 1 (HIF-1A). HIF-1A is also abundantly expressed in the hair follicle.
Let's look up what HIF-1A is ;
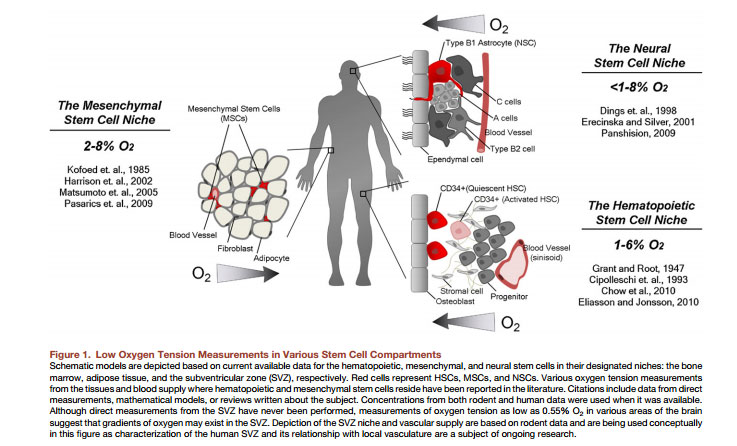
Now what many people don't really know is that the hair follicle is in a moderately to severe hypoxia state. Yes you hear that right, the cells like a hypoxic environment in the hair follicle, they like to bath in low oxygen. Especially the connective tissue which consists of the dermal papilla's too and several stem cells/progenitors. Dermal papilla cells thrive way better under hypoxic conditions too than under normoxic conditions in cell culture. HIF-1A is a regulator which protects this hypoxia state , thus loss of HIF-1A in your hair follicle would completely shred your hair follicle because your cells wouldn't cope with a higher oxygen environment. There are several studies which show this a good short summary;
Another study;
So we know that androgen receptor activation can lead to loss of NOTCH signalling. Does NOTCH signalling have something in common with HIF-1a?
Jup.. It does; http://www.ncbi.nlm.nih.gov/pubmed/16256737
That's right there is evidence in several studies that HIF-1A needs NOTCH signalling to maintain a hypoxia state.
So we could propose now and take this study further that there is a correlation between the 3 of them,
AR> LOSS NOTCH SIGNALLING > LOSS HIF-1A > SENESCENCE PATHWAYS
HIF-1A has major interactions with senescence and P53 etc there are many studies about this;
http://www.nature.com/jid/journal/v1...d2013113a.html
So, perhaps loss of notch signalling due to activation of the androgen receptor leads to loss of HIF-1A in the hair follicle if this would happen, your cells would get stressed as hell (Insane ROS/oxidative stress and possibly DNA DAMAGE) and pathways related to senescence would get activated which in turn would trigger SASP again, an inflammatory response. Who knows.
_____________________________________________________________________________________________________________________________________________________________________________________________________________________
Dermal Papilla! Important niche! Master regulator..
In the 50’s experiments were done by Dr. James Hamilton. He was also the first one who created the term “androgenetic alopecia”. He saw that men who had been castrated before puberty never went bald. Interestingly when he injected testosterone into these castrated men, some suddenly started balding but some did not. Most notably the ones with a family history of baldness started balding.
Why were his experiments so important? Well he showed that baldness is androgen dependent, because castrated men before puberty showed no signs of balding. He also showed that there is a genetic side to the story because when he injected some with testosterone, not everyone started balding. This is also why we call it “androgenetic” alopecia. Because for baldness to occur as we know it you need both androgens + genetics.
To verify this even more Imperato- McGinley studied pseudo-hermaphrodites in the Dominican republic. These pseudo-hermaphrodites were born looking like a girl with female genitals. At puberty however they virilized, developing musculature, a deeper voice and would even develop a penis some of the time. They found out that these pseudo-hermaphrodites had a deficiency of type-2 5-reductase. Then you have people who suffer from androgen insensitivity syndrome. This condition results in a partial or complete inability of cells to respond to androgens. Guess what? Well both of these type people who suffer from this condition never display androgenetic alopecia. This is again a very important observation to make that androgenetic alopecia is androgen dependent. A fun fact is that Merck developed a drug based on the study of Imperato- McGinley; finasteride a type-2 5-reductase inhibitor (5ar2) as we all know it.

Next I want to explain hair follicle cycling in short. A hair follicle cycles as you may know. Everyone experiences this, even your norwood 1 friend. This primarily exists of the following stages; anagen, catagen and lastly telogen, this cycle repeats itself. Anagen is the active growth phase of the hair, catagen is the regression phase and telogen is the resting phase. Now look at the following picture;

What important to know about these cycles is that the hair follicle itself, specifically the micro environment of the hair follicle is able to communicate with the macro environment (surrounding tissue) . For instance in telogen a hair will lack vascularization and blood vessels, not only that it will also lack adipose tissue. In anagen however there is a tremendous increase in vascularization and also adipose tissue. This leads to a thicker dermis in anagen, and a thinner dermis in telogen. Scalp hair follicles reside generally 80-90% in anagen, 1-2% in catagen and 10-15% in telogen. Ok this is enough for general cycling.
Back to androgenetic alopecia again. You should already know that androgenetic alopecia is androgen dependent and has a genetic basis. However for androgenetic alopecia to occur these androgens need to bind to the androgen receptor in the hair follicle. Because as you know people with androgen insensitivity syndrome have hormones but they have impaired androgen receptors and they don’t bald. So where are the androgen receptors located in the hair follicle? They are solely located in the dermal papilla at the base of the hair follicle;

So in order for androgenetic alopecia to occur, androgens which are expressed in the blood need to bind to the androgen receptors located in the dermal papilla. A treatment for example like RU-58841 or CB-03 binds to the androgen receptors there and will stop androgens to attach to these androgen receptors, preventing or slowing androgenetic alopecia. There is evidence that people suffering from androgenetic alopecia have increased androgen receptor expression and increased 5ar2 expression.
In androgenetic alopecia as you guys may very well know too is that with each cycle the hair becomes more miniaturized. The hair becomes thinner, shallower and keeps reducing each cycle until you are basically left with almost nothing. Important to know is that the dermal papilla cells specify hair size, shape and the reduction of them causes a follicular decline (1), (2).
_________________________________________________________________________________________________________________________________________________________________________________________________________________________
Now to the (latest) advancements about the pathology of androgenetic alopecia;
In the study named “Premature Senescence of Balding Dermal Papilla Cells In Vitro Is Associated with p16INK4a Expression”(3). They confirmed as other studies already did(4) that balding dermal papilla cells grow way slower in vitro when compared to non-balding dermal papilla cells. Not only that they found out that balding dermal papilla cells undergo premature senescence. This was associated with the following in the balding dermal papilla cells;
- Increased Expression of p16ink4a
- Increased Expression of pRb
- Expression of senescence-associated β-galactosidase
- Loss of expression of bmi-1
- Increased expression of oxidative stress and dna damage markers like HSP-27, ATM and ATR
Now a study published in November 2014 called “ Androgen Receptor Accelerates Premature Senescence of Human Dermal Papilla Cells in Association with DNA Damage” (5) found ;
- Also a increase of p16ink4a in balding dermal papilla cells and not in non-balding in vitro
- DNA damage accompanied with senescence in the balding dermal papilla cells
- Most importantly when you remove the androgen receptor from the balding dermal papilla cells DNA damage does NOT occur and senescence does NOT occur.
- The dermal papilla cells changed in morphology, they enlarged.
A other study in 2009 called “Proliferation, DNA repair and apoptosis in androgenetic alopecia (6)” found out that in comparison with occipital scalp and frontal balding scalp;
- P53 was overexpressed frontal balding scalp
- P53 showed a inverse correlation with APE-1 (dna damage repair marker)
Another study called "Analysis of microRNA expression profile in 5α -dihydrotestosterone-treated normal human dermal papilla cells" (http://www.papersearch.net/view/detail.asp?detail_key=27731029)
Background: Clinical evidence shows that accumulation of 5 α-dihydrotestosterone(DHT) in dermal papilla cells(DPCs) is implicated in androgenetic alopecia. Objectives: To determine whether DHT affects cell growth, cell cycle arrest, cell death, senescence, and induction of reactive oxygen species(ROS), and whether these effects aremediated by microRNA(miRNA)-dependent mechanisms. Methods: We measured cell viability and cell cycle, detected ROS, and performed senescence-associated β-galactosidase assays in normal human DPCs(nHDPCs). Further, we performed miRNA expression profiling using miRNA microarray to determine whether changes in miRNA expression were associated with the cellular effects of DHT. Results: We found that DHT decreased cell growth by inducing cell death and G2 cell cycle arrest and by increasing ROS production and senescence in nHDPCs. 55 miRNAs were up-regulated and 6 miRNAs were down-regulated in DHT-treated nHDPCs. Bioinformatic analysis showed that putative target genes of these up- and down-regulated miRNAs were involved in cell growth, cell cycle arrest, cell death, senescence, and ROS production.
Conclusion: These results demonstrate that miRNA expression is altered in DHT-treated nHDPCs and suggest a potential mechanism of DHTinduced cell growth repression, cell cycle arrest, cell death, senescence, and ROS induction.
[video=youtube;0klARAxOYiI]https://www.youtube.com/watch?v=0klARAxOYiI[/video]

_____________________________________________________________________________________________________________________________________________________________________________________________________________________________
Short explanation about senescence in general
Read the following picture this is taken from “Cellular senescence: when bad things happen to good cells(7)”. It is NON-Androgenetic Alopecia related but related to general cellular senescence;

I underlined the parts in red that compare with the evidence from the androgenetic alopecia studies. P16(ink4a), pRB, P53 etc. In blue I underlined something that is very typical and extremely important for cellular senescence and that is that these cells release pro-inflammatory factors and even compromise the function of non-senescent healthy cells. Not only that note how they say that it may even lead to a decrease or even a loss in progenitor stem cells. Well as you guys know we are coping with inflammation too in androgenetic alopecia, there are a **** ton of inflammatory factors expressed.
Not only that we have a lack of CD34+ and CD-200 progenitors (http://www.ncbi.nlm.nih.gov/pubmed/21206086)
Bald scalp in men with androgenetic alopecia retains hair follicle stem cells but lacks CD200-rich and CD34-positive hair follicle progenitor cells.
Even the whole morphology of the hair follicle changes.....
1. http://www.ncbi.nlm.nih.gov/pubmed/23487317
2. http://www.nature.com/jid/journal/v1.../5600534a.html
3. http://www.nature.com/jid/journal/v1.../5701147a.html
4. http://www.ncbi.nlm.nih.gov/pubmed/8...ct&holding=npg
5. http://www.plosone.org/article/info%...l.pone.0079434
6. http://www.ncbi.nlm.nih.gov/pubmed/18702626
7. http://www.nature.com/nrm/journal/v8...l/nrm2233.html
______________________________________________________________________________________________________________________________________________________________________________________________________________________________
Further explanation of senescence (why does it happen in general?)
Now for the next part. What trigger can make cells go into a senescent state? What trigger can activate these pathways? Well this can be DNA damage (telomere shortening, single- and double-strand breaks), oncogenic mutations, reactive metabolites (ROS, ceramides, fatty acids, high glucose), increased mTOR activity and proteotoxic stress. As you see in the following picture (This picture most likely doesn't reflect the exact pathway in Androgenetic Alopecia which cause cellular senescence obviously but should give a global view);

Senescence-associated secretory phenotype (SASP) as you see here in the picture above is the result of senescent cells. Senescent cells secrete pro-inflammatory factors. I quote;
“Recent evidence in fibroblasts and epithelial cells has shown that cellular senescence is accompanied by a striking increase in the secretion of 40–80 factors that participate in intercellular signaling. Secretion of this set of factors has been termed the “senescence-associated secretory phenotype”, or SASP. (Inflammatory Networks during Cellular Senescence: Causes and Consequences (4)).
_______________________________________________________________________________________________________________________________________________________________________________________________________________
What factors could be driving the senescence in Androgenetic Alopecia though?
A interesting fact is that androgens act as DNA-damaging agents that generate DNA double-strained breaks (DSBs) which may contribute to the senescence of dermal papilla cells. A other study shows that cultured balding dermal papilla cells secrete more TGF-β (1) and this factor has been shown to stimulate oxidative stress (ROS) (2). Interestingly in normal prostate epithelial cells the androgen receptor has been shown to drive cellular senescence too but without expression of DNA damage or p16ink4a(3). So this reflects the diversity of biological responses the AR can have in relation to senescence in different cell types.
1. http://www.ncbi.nlm.nih.gov/pubmed/1...ct&holding=npg
2. http://www.ncbi.nlm.nih.gov/pubmed/8...ct&holding=npg
3. http://www.ncbi.nlm.nih.gov/pmc/arti...?report=reader
4. http://www.ncbi.nlm.nih.gov/pmc/arti...8/#!po=15.0000
_____________________________________________________________________________________________________________________________________________________________________________________
Trying to propose a link to what causes senescence in Androgenetic Alopecia. Using an Androgenetic Alopecia genetic profile study (don't read to much hypothetical talk);
Using this study; http://www.ncbi.nlm.nih.gov/pmc/articles/PMC4164265/
Microarray analysis of androgenetic and senescent alopecia: Comparison of gene expression shows two distinct profiles
Hair/skin development and function is the most significant physiological function altered in both Androgenetic Alopecia and SA (senescent alopecia), however, the DEGs (differentially expressed genes) in this category differed in the two diseases. Table 1 shows the 34 genes in this category that are differentially regulated in Androgenetic Alopecia that contribute to hair follicle development, morphology and cycling (BARX2, EGFR, INHBA, MSX2, OVOL1, KRTs, KRTAPs, RUNX3 and TIMP3). Many of these genes required for hair follicle homeostasis are significantly under expressed in Androgenetic Alopecia but not in SA compared to normal scalp tissue (Table 1 and Figure S1). Our data (Table 1 & Figure S1)showed that the Androgen Receptor (AR) is up regulated in Androgenetic Alopecia, but not in SA. Previous studies [4] have shown that genetic variability in AR is a prerequisite for the development of early-onset Androgenetic Alopecia. A novel Androgenetic Alopecia susceptibility locus has been identified at 17q21.31 [5]. In our dataset, the DEAD box polypeptide 5 (DDX5), a transcriptional regulator of AR [6] is down regulated in Androgenetic Alopecia and maps to this locus. The most significant pathway altered in Androgenetic Alopecia is Notch Signaling which consists of 29 genes (Table 1) including HES1, Notch2, Notch4 and PROX1 that are known to play a role in cell fate determination [7]. The down regulated genes in this pathway in Androgenetic Alopecia include CNTN1, JAG1, NOTCH2 and PSEN1 and the genes that are up regulated include DTX3, HES and NOTCH4. The expression patterns of Notch signaling pathway genes including Notch 2 and JAG1 were validated by real-time PCR (Figure S1). Jagged1 (JAG1) gene which encodes a ligand for Notch receptor maps to chromosome 20p a susceptibility locus for male-pattern baldness [8]. A reciprocal negative feedback regulation exists between Notch and AR-dependent pathways in the prostate [9]. The activation of AR and the concomitant loss of Notch signaling may be contributing factors to hair follicle miniaturization and may serve as the mechanistic link between prostate cancer and Androgenetic Alopecia. Thus, modulating the Notch signaling pathway in Androgenetic Alopecia may lead to future therapies.
This is the table with differentially expressed genes in androgenetic alopecia with changes of -2 or +2 , basically the ones that stand out the most; http://www.ncbi.nlm.nih.gov/pmc/arti...ort=objectonly

Obviously as said there is evidence again that the androgen receptor is fcked in Androgenetic Alopecia, they found a 2.46 fold increase of this compared to control as you can see in the link just above and in the picture here above where I underlined it in purple. I underlined the Aryl hydrocarbon receptor nuclear translocator-like 2 because that one is the most differentially expressed in this research paper with a fold change of -12.13. This gene encodes a protein that is a co-factor in transcriptional regulation by hypoxia-inducible factor 1 (HIF-1A). We'll come back to this later.
Now they say that the most significant pathway altered in androgenetic alopecia is NOTCH signalling which consists of 29 genes. Furthermore they refer to a other study which found a suspectibility locus for Androgenetic Alopecia at chromosome 20p, and JAGGED1 (JAG1) gene is a ligand for the NOTCH receptor and maps to this. They propose that activation of the androgen receptor leads to loss of NOTCH signalling which results in miniaturization;
Remember DNA damage? Obviously a signal which can lead very well to senescence by pathways like P53/P16/P21 etc like described in this thread.In conclusion, we found that canonical Notch signaling is required for late-stage granular layer differentiation and correct filaggrin processing in the epidermis. Importantly, Notch signaling loss in hair follicle lineages leads to DNA damage response and loss of stem cell characteristics, which is possibly due to aberrant activation of bulge stem cells.
Nonetheless, let's dig a bit further and connect the dots a bit further ourself from the information in this study. We have;
- Altered androgen receptor 2.46 fold
- Altered Aryl hydrocarbon receptor nuclear translocator-like 2 -12.13 fold
- NOTCH signalling most significant pathway altered (29 genes)
Let's propose a hypothesis and try to connect those 3 together. First we are going to look at if NOTCH and the androgen receptor have interactions with each other as proposed;

The AR and Notch receptors play essential roles in the regulation of prostate development and homeostasis. Notch signaling initiates when receptor-bearing cells interact with Notch ligands present in neighboring cells. Notch activation causes an increase in HEY1 expression and HEY1 accumulates in the nucleus repressing AR transcriptional activity. In a reciprocal way, the activation of AR upon androgen binding downregulates the expression of Notch1 receptor and its ligand Jagged1, and upregulates Sel1L, a negative regulator of Notch.
NOTCH is also expressed abundantly in hair follicles. I underlined here evidence that the activation of AR upon androgen binding can for instance downregulate NOTCH signalling and the ligand JAGGED1 which maps to a suspectible locus in Androgenetic Alopecia. I'm not going to go to deep into this, but there are many studies which show androgen receptor interaction with NOTCH, you can look them up yourself if you want.
So yes perhaps in Androgenetic Alopecia because of an overexpressed androgen receptor there is loss of NOTCH signalling. Now we go to the second point , can NOTCH signalling be connected to the Altered Aryl hydrocarbon receptor nuclear translocator-like 2 with a -12.13 fold gene expression in Androgenetic Alopecia? As I this gene encodes a protein that is a co-factor in transcriptional regulation by hypoxia-inducible factor 1 (HIF-1A). HIF-1A is also abundantly expressed in the hair follicle.
Let's look up what HIF-1A is ;
The protein encoded by HIF1 is a bHLH - PAS transcription factor found in mammalian cells growing at low oxygen concentrations. It plays an essential role in cellular and systemic responses to hypoxia.[5] This is one of the class of hypoxia inducible factors, a family that includes Hif1a, Hif2a, and Hif3a. HIF-1 functions as a master regulator of cellular and systemic homeostatic response to hypoxia by activating transcription of many genes, including those involved in energy metabolism, angiogenesis, apoptosis, and other genes whose protein products increase oxygen delivery or facilitate metabolic adaptation to hypoxia.
Now what many people don't really know is that the hair follicle is in a moderately to severe hypoxia state. Yes you hear that right, the cells like a hypoxic environment in the hair follicle, they like to bath in low oxygen. Especially the connective tissue which consists of the dermal papilla's too and several stem cells/progenitors. Dermal papilla cells thrive way better under hypoxic conditions too than under normoxic conditions in cell culture. HIF-1A is a regulator which protects this hypoxia state , thus loss of HIF-1A in your hair follicle would completely shred your hair follicle because your cells wouldn't cope with a higher oxygen environment. There are several studies which show this a good short summary;
Hypoxia is believed to promote an undifferentiated state in several stem and precursor cell populations (Mohyeldin et al., 2010) and our results suggest that the lower stem cell niche of human hair follicles may also be in hypoxic environment. As a portion of CD34+ stem/progenitor cells is located in this hypoxic environment and have been demonstrated to disappear during androgenetic alopecia, we hypothesized that the induction of hypoxia signaling in suboptimal conditions would help maintain hair follicle stem cell functionality and hence prevent alopecia or at least favor neogenesis. Hypoxia signaling is mediated by the hypoxia-inducible transcription factor 1 (HIF1), composed of an αβ heterodimer. The α subunit was reported to be abundantly expressed in human hair follicles (Rosenberger et al. 2007) and is regulated in an oxygen-dependent manner through prolyl-4-hydroxylase-mediated hydroxylation, which mediates proteosomal degradation (Jaakkola et al., 2001).
Another study;
We found that the human dermis is well-oxygenated, the epidermis is modestly hypoxic and portions of some sebaceous glands and hair follicles are moderately to severely hypoxic.
So we know that androgen receptor activation can lead to loss of NOTCH signalling. Does NOTCH signalling have something in common with HIF-1a?
Jup.. It does; http://www.ncbi.nlm.nih.gov/pubmed/16256737
Hypoxia requires notch signaling to maintain the undifferentiated cell state.
In addition to controlling a switch to glycolytic metabolism and induction of erythropoiesis and angiogenesis, hypoxia promotes the undifferentiated cell state in various stem and precursor cell populations. Here, we show that the latter process requires Notch signaling. Hypoxia blocks neuronal and myogenic differentiation in a Notch-dependent manner. Hypoxia activates Notch-responsive promoters and increases expression of Notch direct downstream genes. The Notch intracellular domain interacts with HIF-1alpha, a global regulator of oxygen homeostasis, and HIF-1alpha is recruited to Notch-responsive promoters upon Notch activation under hypoxic conditions. Taken together, these data provide molecular insights into how reduced oxygen levels control the cellular differentiation status and demonstrate a role for Notch in this process.
That's right there is evidence in several studies that HIF-1A needs NOTCH signalling to maintain a hypoxia state.
So we could propose now and take this study further that there is a correlation between the 3 of them,
AR> LOSS NOTCH SIGNALLING > LOSS HIF-1A > SENESCENCE PATHWAYS
HIF-1A has major interactions with senescence and P53 etc there are many studies about this;
http://www.nature.com/jid/journal/v1...d2013113a.html
An interesting corollary to this is that within tissues, oxygen gradients often exist with stem cells residing in the most hypoxic regions (83). It is tempting to think then that these cells, which are known to be more resistant to oxidative stress as a mechanism of self-preservation (84, 85), benefit from their hypoxic environments by avoiding senescence, which would be detrimental to the regenerative capacity of the tissue.
So, perhaps loss of notch signalling due to activation of the androgen receptor leads to loss of HIF-1A in the hair follicle if this would happen, your cells would get stressed as hell (Insane ROS/oxidative stress and possibly DNA DAMAGE) and pathways related to senescence would get activated which in turn would trigger SASP again, an inflammatory response. Who knows.


Notch signaling is widely appreciated to be critical for the maintenance of undifferentiated stem and progenitor cell populations. The observation that hypoxic induction of Notch target genes requires interaction between the Notch intracellular domain and the C-terminal transactivation domain of HIF-1a provided important insight as to how hypoxia may synergize to regulate and promote stemness.
_____________________________________________________________________________________________________________________________________________________________________________________________________________________
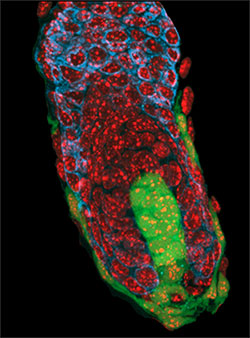
Dermal Papilla! Important niche! Master regulator..
It is possible that the androgen/AR-induced senescence in DPCs may not only lead to diminished DP size but also deregulate the communication between DPCs and hair follicle stem cells to differentiate to progenitor cells.


The hair follicle bulb: The dermal papilla (green cells at the center of the hair bulb) serves as both a physical and chemical niche that regulates the activity of adjacent epithelial progenitor cells (unlabelled except for a red nuclear stain) that produce the hair shaft and its surrounding inner root sheath.
Most of us will experience some degree of hair thinning or loss as we age. In most forms of hair loss, the follicle does not disappear. Instead, it makes shorter and thinner hairs in successive cycles of hair generation until it is converted to a miniaturized “vellus” follicle that makes the tiny unpigmented hairs that remain in most “bald” scalp. The miniaturization process is progressive, with both the dermal papilla and the epithelial hair bulb diminishing in size over several hair cycles. A correlation between the number of cells in the dermal papilla and the size of the hair has been noted during hair thinning, but the question of cause and effect persisted. Does a declining epithelial population cause a `smaller dermal papilla, or does a smaller dermal papilla cause the diminution of the epithelial follicle and the hair shaft? We developed genetically modified mice that allowed us to reduce dermal papilla cell number in adult hair follicles and showed that this causes two of the hallmarks of human hair loss. Successive hair shafts produced by the same follicle are shorter and thinner, and the hair follicle spends longer periods in a quiescent phase before it starts making a new hair.
If we allowed a low level of stochastic DP cell depletion to continue, the mice failed to regenerate their hair coat, the equivalent of balding. However, the pathological cause of DP loss, inducible expression of a cell autonomous toxin specifically in DP cells, was under our control in this work. This allowed us to halt the ongoing cause of hair loss to ask whether diminished follicles were irreversibly damaged. We found that some follicles remained in the quiescent phase and did not generate a new hair. However, others continued to make new hairs and actually restored themselves, increasing the number of DP cells and generating bigger hairs in ensuing cycles. The difference between these two fates was determined by the number of DP cells that remained when the toxin was switched off. A follicle with a few more cells would generate a new hair and restore itself, while a follicle with a few less DP cells no longer contributed new hairs to the pelage.
This suggests good news for those bothered by hair loss. The threshold effect of DP cell number suggests that once the cause of DP cell loss is controlled, therapeutic approaches need only achieve modest success in restoring DP cell number to restore hair cycling. After that, the intrinsic capacity of the hair follicle to restore itself should do the rest of the job. Lest you are tempted to call, let me clearly state that we haven’t found a cure for baldness. However, this work suggests that understanding the mechanisms by which communication between the epithelial and mesenchymal compartments of the follicle regulates DP cell number may be one path to that goal.
