You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
Samumed Sm04554 , What Is Going On With It
- Thread starter wow-wow
- Start date
- Reaction score
- 388
Last news (November 2018)
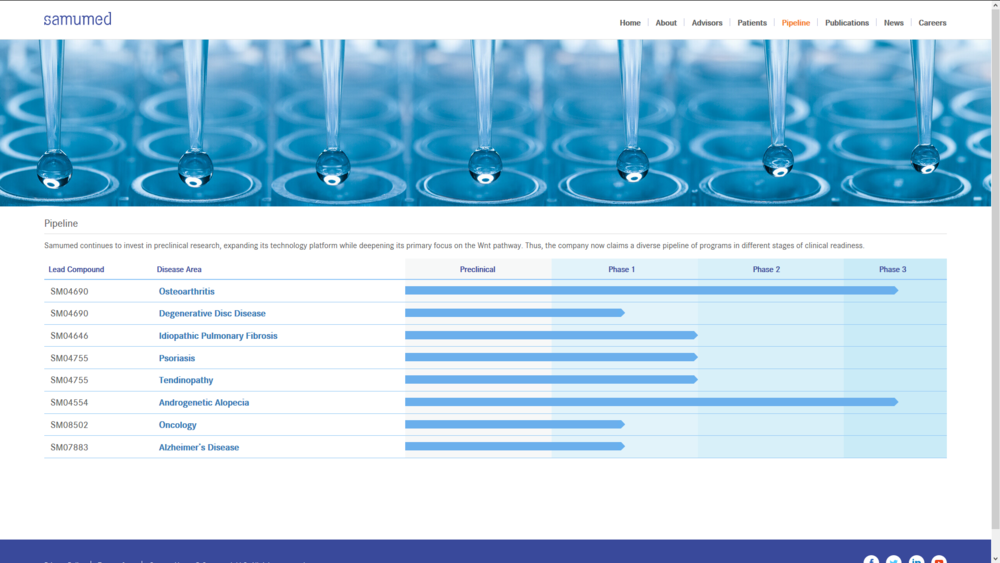
(We anticipate top-line results in the second half of 2020 if successful, would support submission of a marketing application for SM04554 in Turkey.)
" SAN DIEGO, Nov. 19, 2018 (GLOBE NEWSWIRE) -- Samumed, LLC, announced today that it has dosed the first subject in its phase 2/3 trial of SM04554, a topical small molecule Wnt pathway activator, for the treatment of androgenetic alopecia (Androgenetic Alopecia), the most common type of hair loss in men and women. This trial is being conducted as a pivotal study in Turkey and is expected to enroll approximately 625 patients across 11 study sites. This study is conducted under an IND with the FDA. In addition, the Ministry of Health of Turkey has confirmed that the study, if successful, would support submission of a marketing application for SM04554 in Turkey.
“We have shown in phase 2 trials that daily treatment with SM04554 appeared safe and increased follicle counts and non-vellus hair counts compared to vehicle in males with Androgenetic Alopecia after 90 days of exposure,” said Yusuf Yazici, M.D., Chief Medical Officer of Samumed. “In order to build upon these results, this study will evaluate the effect of treatment with topical SM04554 applied daily for 48 weeks on males with Androgenetic Alopecia. We anticipate top-line results in the second half of 2020.”
This trial is a multi-center, randomized, double-blind, placebo-controlled, parallel group study of two doses of topical SM04554 solution (0.15% & 0.25%) applied daily to the scalp of male Androgenetic Alopecia subjects. The trial is a 54-week study, with 48 weeks of treatment followed by 6 weeks of follow-up. The primary endpoint of the study is the baseline-adjusted absolute non-vellus hair count in the target area by phototrichogram analysis at Week 48 compared to vehicle.
Prof. Server Serdaroglu, M.D., principal investigator in this study, said, “The negative psychological effects of androgenetic alopecia are well documented. Currently available treatments have limited efficacy and patients need access to better options. We look forward to contributing to this important clinical study which will examine the effects of SM04554 over the course of one year.”
Samumed has completed two phase 2 studies examining the safety, tolerability, and efficacy of SM04554 for the treatment of Androgenetic Alopecia. These studies demonstrated that treatment with SM04554 appeared to be well-tolerated and safe. Key clinical results have shown that treatment with SM04554 applied daily (90 days) to the scalp resulted in:
(We anticipate top-line results in the second half of 2020 if successful, would support submission of a marketing application for SM04554 in Turkey.)
" SAN DIEGO, Nov. 19, 2018 (GLOBE NEWSWIRE) -- Samumed, LLC, announced today that it has dosed the first subject in its phase 2/3 trial of SM04554, a topical small molecule Wnt pathway activator, for the treatment of androgenetic alopecia (Androgenetic Alopecia), the most common type of hair loss in men and women. This trial is being conducted as a pivotal study in Turkey and is expected to enroll approximately 625 patients across 11 study sites. This study is conducted under an IND with the FDA. In addition, the Ministry of Health of Turkey has confirmed that the study, if successful, would support submission of a marketing application for SM04554 in Turkey.
“We have shown in phase 2 trials that daily treatment with SM04554 appeared safe and increased follicle counts and non-vellus hair counts compared to vehicle in males with Androgenetic Alopecia after 90 days of exposure,” said Yusuf Yazici, M.D., Chief Medical Officer of Samumed. “In order to build upon these results, this study will evaluate the effect of treatment with topical SM04554 applied daily for 48 weeks on males with Androgenetic Alopecia. We anticipate top-line results in the second half of 2020.”
This trial is a multi-center, randomized, double-blind, placebo-controlled, parallel group study of two doses of topical SM04554 solution (0.15% & 0.25%) applied daily to the scalp of male Androgenetic Alopecia subjects. The trial is a 54-week study, with 48 weeks of treatment followed by 6 weeks of follow-up. The primary endpoint of the study is the baseline-adjusted absolute non-vellus hair count in the target area by phototrichogram analysis at Week 48 compared to vehicle.
Prof. Server Serdaroglu, M.D., principal investigator in this study, said, “The negative psychological effects of androgenetic alopecia are well documented. Currently available treatments have limited efficacy and patients need access to better options. We look forward to contributing to this important clinical study which will examine the effects of SM04554 over the course of one year.”
Samumed has completed two phase 2 studies examining the safety, tolerability, and efficacy of SM04554 for the treatment of Androgenetic Alopecia. These studies demonstrated that treatment with SM04554 appeared to be well-tolerated and safe. Key clinical results have shown that treatment with SM04554 applied daily (90 days) to the scalp resulted in:
- Significantly increased non-vellus hair counts and non-vellus hair density with treatment (0.15% SM04554) compared to vehicle (NCT02275351).
- Significantly increased follicle counts in both treatment groups (0.15% and 0.25% SM04554) compared to vehicle (NCT02503137). "
Last edited:
HairlossCurse
Member
- Reaction score
- 39
They say that it increased non-velus count but give no number, e.g. 5% increase, 10% increase. Also, no photos even after completing trials so I would be skeptical about the whole thing. Also very vague about how it works, mention of WNT, but there are many different types of WNTs etc.
- Reaction score
- 388
They say that it increased non-velus count but give no number, e.g. 5% increase, 10% increase. Also, no photos even after completing trials so I would be skeptical about the whole thing. Also very vague about how it works, mention of WNT, but there are many different types of WNTs etc.
Not true ! They have already given the results. 2 differents phase II (only on a period of 90 days).
https://www.hairlosstalk.com/intera...-2-results-safety-and-biopsy-outcomes.104093/
- Reaction score
- 1,242
https://www.fiercebiotech.com/biotech/conversation-osman-kibar-ceo-samumed
" It's alopecia treatment is close behind, with approval anticipated in late 2021 or early 2022"
" It's alopecia treatment is close behind, with approval anticipated in late 2021 or early 2022"
Sanchez1234
Experienced Member
- Reaction score
- 311
You can buy it from Wuhan already since the molecule is published
- Reaction score
- 15
Still unsure if this treatment is worth or not. It seems it could cause a risk of cancer since it targets WNT (https://www.ncbi.nlm.nih.gov/pubmed/28647886).
Also, from their Fierce Biotech interview, they also said that it's targetting CLK and DYRK, which are related to cancer too (https://www.ncbi.nlm.nih.gov/pubmed/29769258 & https://www.ncbi.nlm.nih.gov/pubmed/28554575)
Because of that, and the fact that it's still not a cure, I don't know if we really should wait for this.
(And their phase III will only lasts 1 year, so they're not going to see any safety issues about cancer in this timeframe)
Also, from their Fierce Biotech interview, they also said that it's targetting CLK and DYRK, which are related to cancer too (https://www.ncbi.nlm.nih.gov/pubmed/29769258 & https://www.ncbi.nlm.nih.gov/pubmed/28554575)
Because of that, and the fact that it's still not a cure, I don't know if we really should wait for this.
(And their phase III will only lasts 1 year, so they're not going to see any safety issues about cancer in this timeframe)
- Reaction score
- 1,052
Still unsure if this treatment is worth or not. It seems it could cause a risk of cancer since it targets WNT (https://www.ncbi.nlm.nih.gov/pubmed/28647886).
Also, from their Fierce Biotech interview, they also said that it's targetting CLK and DYRK, which are related to cancer too (https://www.ncbi.nlm.nih.gov/pubmed/29769258 & https://www.ncbi.nlm.nih.gov/pubmed/28554575)
Because of that, and the fact that it's still not a cure, I don't know if we really should wait for this.
(And their phase III will only lasts 1 year, so they're not going to see any safety issues about cancer in this timeframe)
don't think so, there are products targeting wnt pathway and that are sold by big pharma group such as deltracin or Neoptide hair lotion or sebovalis. IF I remember well lactoferin also targets it and there are countless products containing it. All in all if a products becomes available on the market, and if this is the case for SM, that obviously means it's safe for human use and for its purpose. The only thing we can do is wait = )
- Reaction score
- 15
don't think so, there are products targeting wnt pathway and that are sold by big pharma group such as deltracin or Neoptide hair lotion or sebovalis. IF I remember well lactoferin also targets it and there are countless products containing it. All in all if a products becomes available on the market, and if this is the case for SM, that obviously means it's safe for human use and for its purpose. The only thing we can do is wait = )
Well, honestly I'm not sure if we can trust big pharma at 100% about long-term risks, just need to see hormonal treatment such as finasteride, androcure etc. I wouldn't call it very safe, but they're still sold. But well, really really hope you're right and I'm just paranoïd, because this product seems pretty interesting (especially the fact that it may be the first treatment causing follicular neogenesis).
Anyway, since you're talking about it, given that Neoptide seems to target the WNT pathway too, did someone used it with results? It could allow us to use it instead of SM04554 which still need few years before commercialization.
- Reaction score
- 1,242
https://www.folliclethought.com/samumed-sm04554-trial-in-turkey/#comment-25899
Turkey release first, would be perfect for Europeans. Get a hair transplant and SM at a low-cost price. Hope this sh*t works at all
Turkey release first, would be perfect for Europeans. Get a hair transplant and SM at a low-cost price. Hope this sh*t works at all
Sanchez1234
Experienced Member
- Reaction score
- 311
It went through the safety trials + its already available from China (like RU CB etc).
- Reaction score
- 1,242
No FDA, if they succeed they could immediately release the product in a country very close/within Europe and get the procedure done while having the whole European market next door. People that get a hair transplant in Turkey would get this done too and it would be much cheaper to handle it then it the US.Still don't get why they're doing the trial in Turkey, unless they're basically just going to bring it to market and see how effective it is widespread before bothering with US approval
- Reaction score
- 0
where can I buy it?It went through the safety trials + its already available from China (like RU CB etc).
Still don't get why they're doing the trial in Turkey, unless they're basically just going to bring it to market and see how effective it is widespread before bothering with US approval
It's cheaper in Turkey. Pretty sure they can still use the trial for fda approval
- Reaction score
- 338
Comes out in turkey but not yet in US -> Buy online for marked up price.It's cheaper in Turkey. Pretty sure they can still use the trial for fda approval
