- Reaction score
- 950
iTRAQ-Based Quantitative Proteomic Comparison of Early- and Late-Passage Human Dermal Papilla Cell Secretome in Relation to Inducing Hair Follicle Regeneration
Excerpts:
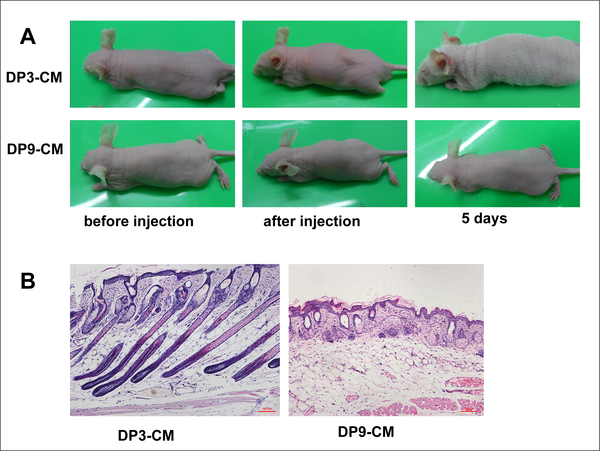
Fig 2. In vivo hair follicle induction by different passage DPC culture media.
(A)Passage 3, but not passage 9 DPC-CM, induces hair generation on NU/NU mouse backs after 5 days. (B) Skin histology reveals HF structures in mice injected with passage 3 DPC-CM compared with mice injected with passage 9 DPC-CM.
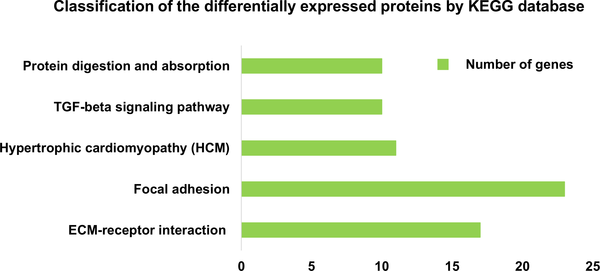
Fig 4. Definition of differentially expressed proteins based on KEGG analysis.
Classification of secreted proteins differentially expressed between passage 3 DPC-CM and passage 9 DPC-CM, according to the KEGG database (P<0.01).
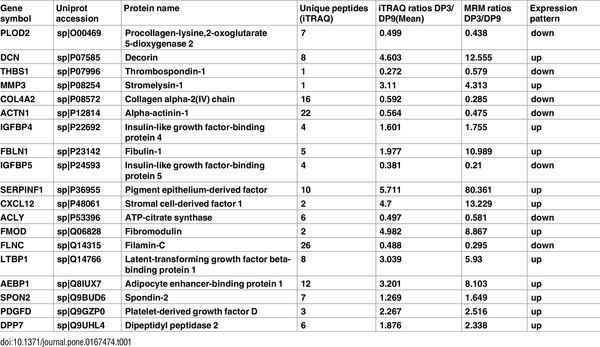
Table 1. iTRAQ-based quantification compared with MRM-based quantification
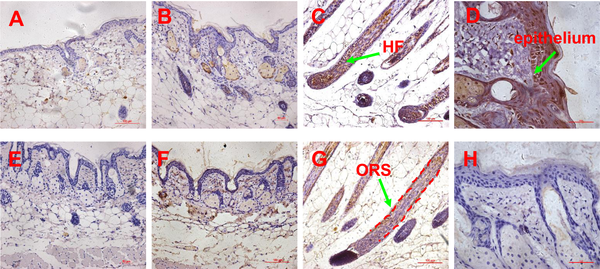
Fig 6. Expression pattern of SDF1 and MMP3 proteins determined by immunohistochemistry.
(A-B) the expression of SDF1 in the skin of untreated and passage 9 DPC-CM injected mice (20x objective), (C) the expression of SDF1 in the skin of passage 3 DPC-CM injected mice (20x objective), (D) the expression of SDF1 in the epithelium of skin form passage 3 DPC-CM injected mice (40x objective); (E-F) the expression of MMP3 in the skin of untreated and passage 9 DPC-CM injected mice (20x objective), (G) the expression of MMP3 in the ORS of skin from passage 3 DPC-CM injected mice (20x objective), (H) the expression of MMP3 in the epithelium of passage 3 DPC-CM injected mice skin (40x objective).
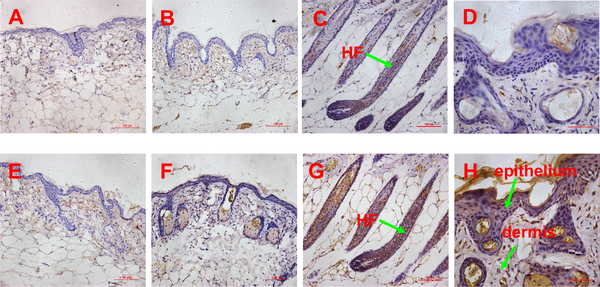
Fig 7. Expression pattern of biglycan and LTBP1 proteins determined by immunohistochemistry.
(A-B) the expression of biglycan in the skin of untreated and passage 9 DPC-CM injected mice (20x objective), (C) the expression of biglycan in the skin of passage 3 DPC-CM injected mice (20x objective), (D) the expression of biglycan in the epithelium of skin form passage 3 DPC-CM injected mice (40x objective); (E-F) the expression of LTBP1 in the skin of untreated and passage 9 DPC-CM injected mice (20x objective), (G) the expression of LTBP1 in the ORS of skin from passage 3 DPC-CM injected mice (20x objective), (H) the expression of LTBP1 in the epithelium of skin from passage 3 DPC-CM injected mice (40x objective).
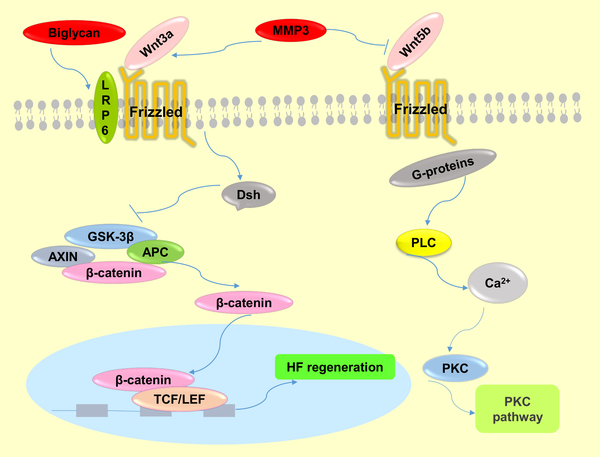
Fig 8. The molecular mechanisms of Wnt signaling pathway regulation by MMP3 and biglycan.
MMP3 activates the canonical Wnt signaling pathway by cooperating with Wnt3a or antagonizing Wnt5b function, which is a non-canonical Wnt ligand and inhibitor of Wnt/β-catenin signaling. Biglycan enhances Wnt-induced β-catenin/TCF-mediated transcriptional activity via the LRP6 receptor.
Abstract
Alopecia is an exceedingly prevalent problem that lacks effective therapy. Recently, research has focused on early-passage dermal papilla cells (DPCs), which have hair inducing activity both in vivo and in vitro. Our previous study indicated that factors secreted from early-passage DPCs contribute to hair follicle (HF) regeneration. To identify which factors are responsible for HF regeneration and why late-passage DPCs lose this potential, we collected 48-h-culture medium (CM) from both of passage 3 and 9 DPCs, and subcutaneously injected the DPC-CM into NU/NU mice. Passage 3 DPC-CM induced HF regeneration, based on the emergence of a white hair coat, but passage 9 DPC-CM did not. In order to identify the key factors responsible for hair induction, CM from passage 3 and 9 DPCs was analyzed by iTRAQ-based quantitative proteomic technology. We identified 1360 proteins, of which 213 proteins were differentially expressed between CM from early-passage vs. late-passage DPCs, including SDF1, MMP3, biglycan and LTBP1. Further analysis indicated that the differentially-expressed proteins regulated the Wnt, TGF-β and BMP signaling pathways, which directly and indirectly participate in HF morphogenesis and regeneration. Subsequently, we selected 19 proteins for further verification by multiple reaction monitoring (MRM) between the two types of CM. These results indicate DPC-secreted proteins play important roles in HF regeneration, with SDF1, MMP3, biglycan, and LTBP1 being potential key inductive factors secreted by dermal papilla cells in the regeneration of hair follicles.
Excerpts:
In order to determine whether DPC-secreted proteins induce HF regeneration, we collected passage 3 and 9 DPC-CM, concentrated the CM through an Amicon filter, and subcutaneously injected the concentrated (2 mg/ml) CM into NU/NU mice. For passage 3 DPC-CM, a white hair coat emerged about 3 days after injection, and by 5 days, the hair fibers reached 1 cm in length, followed by hair loss after an additional 3 or 4 days. In contrast, backs of control mice, injected with passage 9 DPC-CM, remained hairless. (Fig 2A). Skin histology revealed hair follicle structures in passage 3 DPC-CM-injected mice (Fig 2B).
Fig 2. In vivo hair follicle induction by different passage DPC culture media.
(A)Passage 3, but not passage 9 DPC-CM, induces hair generation on NU/NU mouse backs after 5 days. (B) Skin histology reveals HF structures in mice injected with passage 3 DPC-CM compared with mice injected with passage 9 DPC-CM.
Fig 4. Definition of differentially expressed proteins based on KEGG analysis.
Classification of secreted proteins differentially expressed between passage 3 DPC-CM and passage 9 DPC-CM, according to the KEGG database (P<0.01).
Of the 213 proteins that showed a significant difference by iTRAQ analysis, we selected 19 proteins for analysis by MRM. The detailed information from the iTRAQ analysis, including the fold changes, is supplied in S9 Table. The MRM transitions of each targeted peptide and CE (Collision Energy) is detailed in S10 Table, and the MRM ratio is detailed in S11 Table. As a result, the 19 proteins were successfully quantified, and all the target proteins showed the same trend as when quantified by iTRAQ (Table 1).
Table 1. iTRAQ-based quantification compared with MRM-based quantification
In order to confirm SDF1, MMP3 and LTBP1 to be potential key factors in HF regeneration, tissues of passage 3 DPC-CM-injected, passage 9 DPC-CM injected and untreated mice were obtained to determine their expression patterns by immunohistochemistry. Also, the expression pattern of biglycan known to play key roles in many cellular signaling pathways involved in hair follicle biology was also detected. In the skin of passage 9 DPC-CM injected mice and untreated mice, SDF1 (Fig 6A and 6B), MMP3 (Fig 6E and 6F), biglycan (Fig 7A and 7B) and LTBP1 (Fig 7E and 7F) were rarely expressed. Interestingly, SDF1 (Fig 6C) and biglycan (Fig 7C), as well as LTBP1 (Fig 7G) proteins were all expressed in hair follicles, with the highest expression of SDF1 (Fig 6D) being located to the epithelial cells in the skin from passage 3 DPC-CM-injected mice. In addition, MMP3 was expressed only in the ORS of passage 3 DPC-CM-injected mice skin (Fig 6G), whereas LTBP1 was strongly expressed in epithelial tissue and dermal cells (Fig 7H).
Fig 6. Expression pattern of SDF1 and MMP3 proteins determined by immunohistochemistry.
(A-B) the expression of SDF1 in the skin of untreated and passage 9 DPC-CM injected mice (20x objective), (C) the expression of SDF1 in the skin of passage 3 DPC-CM injected mice (20x objective), (D) the expression of SDF1 in the epithelium of skin form passage 3 DPC-CM injected mice (40x objective); (E-F) the expression of MMP3 in the skin of untreated and passage 9 DPC-CM injected mice (20x objective), (G) the expression of MMP3 in the ORS of skin from passage 3 DPC-CM injected mice (20x objective), (H) the expression of MMP3 in the epithelium of passage 3 DPC-CM injected mice skin (40x objective).
Fig 7. Expression pattern of biglycan and LTBP1 proteins determined by immunohistochemistry.
(A-B) the expression of biglycan in the skin of untreated and passage 9 DPC-CM injected mice (20x objective), (C) the expression of biglycan in the skin of passage 3 DPC-CM injected mice (20x objective), (D) the expression of biglycan in the epithelium of skin form passage 3 DPC-CM injected mice (40x objective); (E-F) the expression of LTBP1 in the skin of untreated and passage 9 DPC-CM injected mice (20x objective), (G) the expression of LTBP1 in the ORS of skin from passage 3 DPC-CM injected mice (20x objective), (H) the expression of LTBP1 in the epithelium of skin from passage 3 DPC-CM injected mice (40x objective).
Among the most enriched pathways (P<0.01), the TGF-β signaling pathway has been shown to be essential for regeneration, as it activates the Smad2/3 pathway in HF stem cells, which is crucial for avoiding delayed regeneration [3].
In this study, we focus on identifying the key factors responsible for hair inductive capacity. HF regeneration involves BMP antagonism and subsequent activation of Wnt and other underlying pathways. The initial steps of regeneration involve crosstalk between quiescent epithelial stem cells and mesenchymal dermal papilla, forming a repressive environment for BMP signaling [3, 21]. In our proteomic analysis, we searched for factors involved in the Wnt and BMP pathways and identified SDF1, MMP3 and biglycan, which participate in the Wnt signaling pathway, as well as LTBP1, which participates in the BMP signaling pathway.
We identified stromal cell-derived factor 1 (SDF1, also known as CXCL12) as being one of the most overexpressed in early-passage DPCs, compared to late passage DPCs. SDF-1 is a ligand for CXCR4, is a powerful chemoattractant for stem cells, and plays an important role in several developmental and regenerative phenomena, such as cardiogenesis, neovascularization, hematopoiesis, and hepatic development [22]. SDF-1 is localized to the hair follicle dermal papilla (DP), but is excluded from the epidermis. CXCR4 is specifically expressed in the hair follicle dermal papilla, where it is localized to a few epidermal cells at the mouth of developing hair follicles. The close juxtaposition and complementary nature of CXCR4 and SDF-1 expression patterns suggest that SDF-1/CXCR4 signaling might play a role in HF development [23]. However, in our induced HF regeneration, compared with passage 9 DPC-CM injected mice, SDF1 was strongly expressed not only in the HF, but also in the epithelium of passage 3 DPC-CM-injected mice. Those findings indicate that SDF1 may be a key factor for initiating hair follicle regeneration. A recent report suggests that SDF-1 promotes epidermal stem cell (ESC) migration and accelerates wound healing in vivo [22]. Based on previous studies, SDF1 has the potential to regulate HF regeneration. However, the molecular mechanisms have not been demonstrated. Based on the interaction between SDF1/CXCR4 signaling and Wnt/β-catenin in some cancer cells and neural progenitors [24–26] and the important role in HF initiation as well as primary hair placode maintenance of Wnt/β-catenin signaling pathway, we hypothesize that SDF1 may participate in HF regeneration by regulating Wnt/β-catenin signaling.
Another identified DPC-secreted factor, matrix metallopeptidase 3 (MMP3), belongs to the matrix metalloproteinase (MMP) family of proteases that has been implicated in a large variety of physiological and pathological conditions associated with tissue remodeling [27]. Although the function of MMP3 in HF regeneration has not yet been reported, MMP can modify the microenvironment of stem cells in bone, and MMP3 has been shown to have an impact on the maintenance of adult epithelial stem cells [28], implying MMP3, which we show is increased in early passage DPCs, has the potency to modify the hair follicle stem cell (HFSC) microenvironment “niche”, which plays a crucial role in cell fate decision. MMP3 could affect adult epithelial stem cells though activating Wnt/β-catenin signaling by antagonizing the function of Wnt5b (Fig 8), a non-canonical Wnt ligand and inhibitor of Wnt/β-catenin signaling [29]. Another report showed that MMP3 cooperates with Wnt3a to increase the transcriptional activity of β–catenin in C57MG cells (Fig 8) [27]. Additionally, a recent study suggested that MMPs serve as paracrine factors to activate Wnt signaling in mesenchymal stem cells (MSCs) [30]. It is well known that the Wnt signaling pathway plays a crucial role in the maintenance and proliferation of HFSC reservoirs [3]. In addition, MMP3 was highly expressed in the ORS of passage 3 DPC-CM-injected mice, which is close to the bulge where hair follicle stem cells (HFSCs) are located. Taken together, we speculate that MMP3 participates in the process of HF regeneration by activating Wnt/β–catenin in HFSCs (Fig 8).
A third important factor is biglycan, a member of the small leucine-rich proteoglycan family, and an important structural component of the ECM [31, 32]. Biglycan functions as a signaling molecule released from the ECM. Proteoglycans are known to play key roles in many cellular signaling pathways involved in hair follicle biology [33]. Interestingly, although the expression level of biglycan was increased in the skin of passage 3 DPC-CM-injected mice and was highly expressed in the induction of HFs, it was rarely expressed in the skin of passage 9 DPC-CM injected mice in this study, consistent with a prior demonstration that the DP contains a high level of biglycan [33], and suggests that biglycan is involved in key functions of HF biology. Furthermore, both biglycan proteoglycan and biglycan core protein enhance Wnt-induced β-catenin/TCF-mediated transcriptional activity via the LRP6 receptor (Fig 8) [31]. Recently, additional research has demonstrated that biglycan, as a component of the ECM, mediates suture expansion osteogenesis through the Wnt/β-catenin signaling-activated Runx2 pathway [32]. Therefore, it is possible that DPC secretion of biglycan impacts HF regeneration by also regulating the Wnt/β-catenin signaling pathway (Fig 8).
Fig 8. The molecular mechanisms of Wnt signaling pathway regulation by MMP3 and biglycan.
MMP3 activates the canonical Wnt signaling pathway by cooperating with Wnt3a or antagonizing Wnt5b function, which is a non-canonical Wnt ligand and inhibitor of Wnt/β-catenin signaling. Biglycan enhances Wnt-induced β-catenin/TCF-mediated transcriptional activity via the LRP6 receptor.
Although, we do not discuss the direct regulators involved in BMP antagonists in our study, we confirmed that latent TGF-β-binding protein 1 (LTBP1) is over-expressed in passage 3 DPC-CM. In addition, we show that LTBP1 was not expressed in the skin of passage 9 DPC-CM injected mice, but was strongly expressed in the regenerated HF, epithelium and dermis. LTBP-1 covalently binds to the small latent TGF-β complex and regulates its function, presumably via interaction with the ECM (34). Several prior reports illustrate that LTBP1 is a positive modulator of TGF-β1 activation [34–36], and is considered an antagonist of BMP4 signaling through activating TGF-β1 signaling via the TGF-β1 type I receptor [37]. Dermal papillae produce a paracrine TGF-β2 signal to activate canonical TGF-β-mediated transcription and induce proliferation in quiescent HFSCs, and propels them into a tissue-regenerating mode. Additionally, BMP signaling is prolonged when HFSCs cannot respond to TGF-β2. Tmeff1, a relevant TGF-β2 target gene, is an antagonist of the BMP pathway. Intriguingly, LTBP1 increases TGF-β1 and TGF-β2 at the same time in glioma cells [34].Thus, LTBP1 could inhibit BMP signaling by activating the TGF-β signaling pathway.
Conclusion
Our results suggest that DPCs regulate hair follicle regeneration in paracrine fashion by secreting SDF1, MMP3 and biglycan, to activate Wnt/β-catenin signaling, and LTBP1 to block BMP signaling, as part of establishing a microenvironment for the induction of HF regeneration.
