Where did you see Follica's phase 2 results? Post a link to the source.
Not who ur asking lol, but I think this is it. I dont think this what he saw.
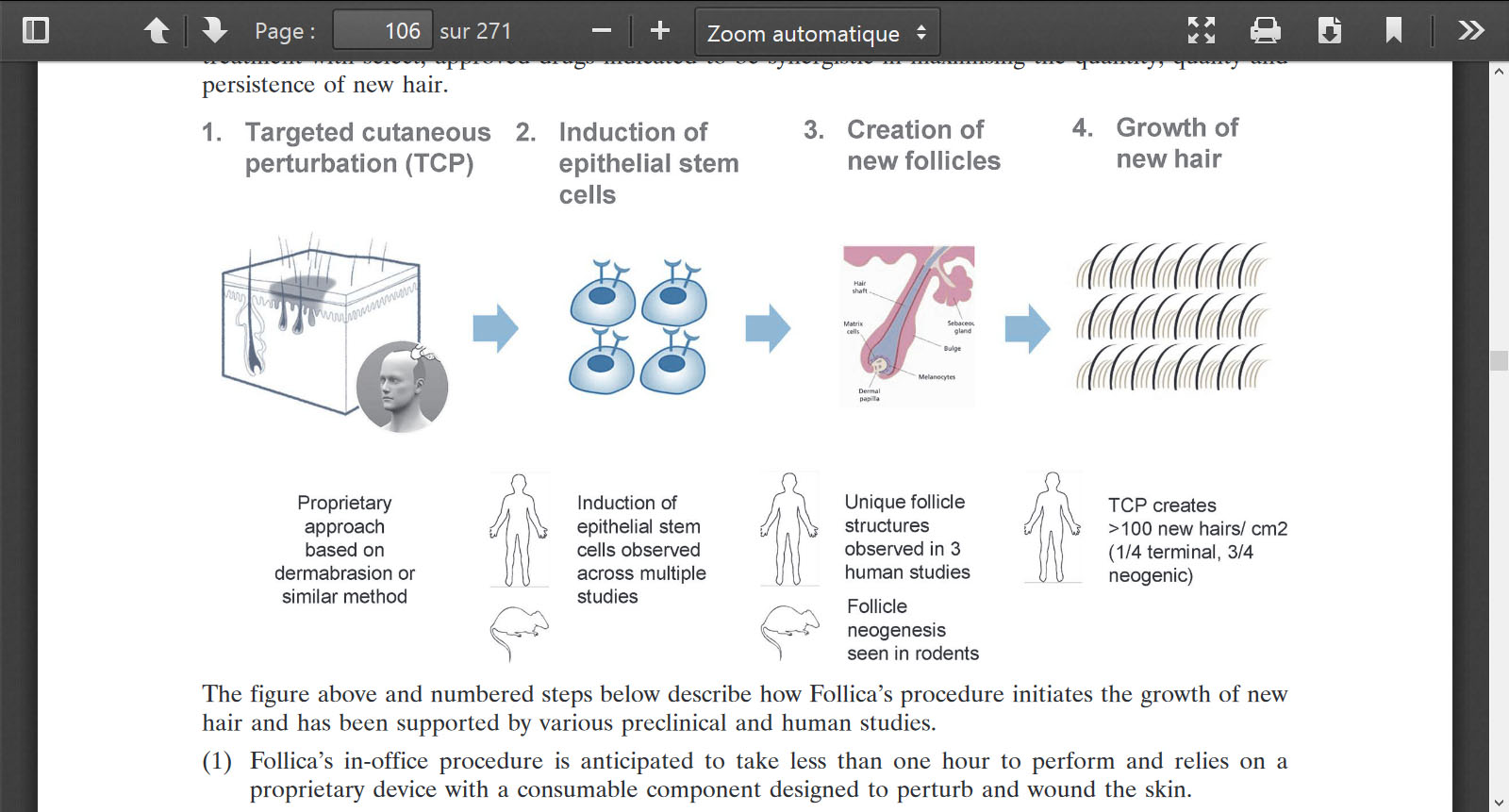
8.1 STUDY PROTOCOL
[00637]
The following protocol describes a Phase Ila clinical study that evaluated the effect of dermabrasion and post-perturbation treatment with a hydrogel (comprising Carbomer (Carbopol 980), glycerol, sodium hydroxide, methylparaben, propylparaben, and purified water) on (i) the formation of neogenic-like or activated or stimulated hair follicle structures (e.g., NL, PEL and PELA as described in Section 5.8.4.1 supra) and (ii) hair growth.
[00638] Diagnosis and main criteria for inclusion are Caucasian males 20-65 years of age, providing written informed consent, who have androgenetic alopecia with the presence of a vertex transition zone defined as an area possessing both terminal and miniaturized hairs, Hamilton-Norwood type 3V, 4, 5, 5A, or 5V, with a vertex area large enough to accommodate both treatment sites, and Fitzpatrick skin type 1-4.
8.1.1 Efficacy objectives
[00639] Primary. To assess changes from Baseline to Day 84 in the number of photographically detected hairs in the target analysis area of the skin of subjects treated with controlled cutaneous perturbation using dermabrasion (DA - a more superficial integumental perturbation) plus the topical application of a hydrogel.
[00640] Secondary, (i) To assess the changes from Baseline to Day 168 in the number of photographically detected hairs in the target analysis area of the skin of subjects treated with controlled cutaneous perturbation using dermabrasion (DA) plus the topical application of hydrogel; (ii) To assess the number of histologically detected neogenic-like hair follicles and other hair follicle structures of interest in biopsies taken on Day 14 from subjects treated with controlled cutaneous perturbation using dermabrasion (DA) plus topical hydrogel; (iii) To assess the number of histologically detected hair follicles in biopsies taken on Day 168 from the site of the first biopsy (allowed to heal by secondary intentions) in subjects treated with controlled cutaneous perturbation using a 4 mm punch biopsy plus topical hydrogel.
[00641] Exploratory, (i) To assess a) at Day 84 and b) at Day 168 the number of photographically detected hairs in subjects treated with controlled cutaneous perturbation using a 4 mm punch biopsy plus the topical application of hydrogel; (ii) To assess a) at Day 84 and b) at Day 168 the h a i r shaft thickness of photographically detected hairs induced by treatment with controlled cutaneous perturbation using a 4 mm punch biopsy plus the topical application of hydrogel; (iii) To assess changes a) from Baseline to Day 84 and b) from Baseline to Day 168 in hair shaft thickness of photographically detected hairs after treatment with controlled cutaneous perturbation with dermabrasion (DA) plus the topical application of hydrogel; (iv) the histological characteristics in a second skin punch biopsy on Day 168.
[00642] Photographic fields of measurement include the Total Analysis Area, which is a 1.13 cm2 circular region in an area that is dermabraded on Day 0, treated with hydrogel, undergoes a 4 mm punch biopsy on Day 14, and then receives additional treatment with hydrogel. Within the Total Analysis Area, there is the Circular Biopsy Area, which is a 0.13 cm2circular region that undergoes a 4 mm punch biopsy, and the Target Analysis Area, which is the Total Analysis Area minus the Circular Biopsy Area, which is a 1.00 cm2 circular region that has received only dermabrasion.
8.1.2 SAFETY OBJECTIVES
[00643] To assess the safety and tolerability of hydrogel in the setting of controlled cutaneous perturbation (dermabrasion and punch biopsy).
[00644] The safety and tolerability of hydrogel gel applied topically and epidermal disruption by dermabrasion and punch biopsies will be monitored through the collection of data from targeted examination of the treated scalp sites and the reporting of adverse events (AEs). Visits on Days 1, 2, 3, 12, 15, 17, 18, 19, 20 and 182 are safety visits to assess the sites (although visits on Days 3, 18, 19 and 20 may be replaced by calls if they fall on the weekend). Adverse events will also be reported at safety phone calls on Days 1 12 and 140. In addition liver and renal function, Hgb- AlC, and urinalysis will be performed at screening and on Days 182. A physical examination will also be performed at screening and Day 182. Vital signs and ECG will be performed at screening. On days 0, 14 168 (when dermabrasion and punch biopsies are performed) and 182 (end of study or at early termination), vital signs will be repeated.
8.1.3 TREATMENT METHODS
[00645] Treatment in this study consists of two treatment modalities:
[00646] 1. Physical perturbation (Dermabrasion and full thickness excision)
[00647] 2. Pharmacological intervention
[00648] Subjects are scheduled to receive hydrogel for 31 days: treatment period 1 (Day 0 until 2 days prior to punch biopsy 1 [Day 11]) and treatment period 2 (Day 17 until end of treatment [Day 35]). The dose of hydrogel is an approximate volume of 0.1 mL applied twice daily to two sites, for a total intended volume of 0.4 mL. Due to droplet size variability, this translates to an actual total volume range of 0.27 to 0.88 mL. [00649] The planned duration of the study per subject is 196 days, comprising a 14 day screening period, and 182 days of treatment and follow-up. The planned total duration of the study is approximately 12 months.
[00650] Once eligibility is confirmed (Day -6 / 0), subjects will have Baseline photography that includes a pin-point tattoo and hair dye. Two sites will be assigned, each measuring 1.5 cm x 1.5 cm and designated right (R) and left (L) with a minimal distance of 2 cm, identified in transitional areas of the balding vertex scalp, which has a very low density of terminal hairs. The hair density of the 2 sites should be as similar as is possible.
[00651] Dermabrasion of two sites per subject will be carried out using a hand-held dermabrader with a standard grit diamond fraise to achieve pinpoint capillary bleeding (estimated depth 100 microns, and therefore not anticipated to cause scarring). After dermabrasion the hydrogel will be applied to the two sites.
[00652] On Day 14, the two dermabraded sites will receive a full thickness 4 mm skin punch biopsy that is allowed to heal by secondary intention without occlusion. In addition to detecting neogenic-like follicles and possibly other activated, stimulated, or reorganized follicular structures of interest (e.g., PEL and PELA as described in Section 5.8.4.1 supra) following treatment with DA (a more superficial perturbation) and hydrogel, the 4 mm punch biopsies also provide a deeper perturbation that will be tested with hydrogel for the induction of neogenic-like follicles and other follicular structures of interest
[00653] Subjects will return after 3 months (Day 84) and 6 months (Day 168) for repeat photographic and clinical evaluations. Monthly safety follow-up phone calls will be performed on Days 112 and 140.
[00654] On Day 168 a second skin punch biopsy will be performed over the 2 dermabraded sites where there was a first punch biopsy on Day 14. In addition to providing samples for an analysis of efficacy, any scar tissue formed from the 4 mm punch biopsies on Day 14 will be excised by this second biopsy on Day 168, which will be closed by sutures. At the discretion of the investigator and if in accordance with the subject's wish, the Day 168 punch of the dermabraded sites will be a 5 mm or 6 mm skin biopsy (or elliptical biopsy by hand, or with an excisor, of a similar size) in order to assure scar removal and photography tattoo removal. The sutures are scheduled to be removed at a safety follow-up 2 weeks later (Day 182).
8.2 RESULTS [00655J A clinical study was carried out in accordance with the protocol described above. A summary of the demographics and characteristics of subjects treated with integumental perturbation and a hydrogel containing no active substance is shown in Table 8.
[00656] It was found that, for subjects treated with dermabrasion plus hydrogel:
• From baseline to Day 84 (3 months), target area hair counts (also referred to as "TAHC") of all hair show substantial increases that are statistically significant (see Table 9). This change in counts of all hair from baseline is maintained through the last day of measurement, Day 168 (6 months; See Table 10) and the change is statistically significant on Day 168.
• From baseline to Day 84 (3 months), target area hair count of only nonvellus-sized hairs, which have hair shafts 30 microns and greater in diameter (widths measured photographically), shows substantial increases that are statistically significant (see Table 11). This change in counts of nonvellus-sized hair from baseline is not maintained through the last day of measurement, Day 168 (6 months; See Table 12).
• From baseline to Day 84 (3 months), target area hair count of only vellus-sized hairs, which have hair shafts less than 30 microns in diameter (widths measured photographically), shows small increases (see Table 13). From baseline to the last day of measurement, Day 168 (6 months), target area hair count of only vellus-sized hairs shows substantial increases that are large (see Table 14).
[00657] As shown in Tables 15 and 16, the sustained positive change in all hair at the 6 month time point is comprised in large part by the striking increase in hair follicles with shafts between 10-20 microns in diameter (widths measured photographically). The increase in follicles with shafts between 20-30 microns has a small contribution to this overall positive change (see Table 14).
[00658] In dermabraded areas of skin, induction of neogenic-like hair follicles and activated, stimulated, or reorganized pre-existing (including vellus-sized) follicles was measured as detected and analyzed by skin biopsy analysis at Day 14. In contrast to what is generally found in unwounded scalp skin, the controlled perturbation in this study 1) induced neogenic-like follicles and 2) placed preexisting follicles into a reorganized and activated state. The structures of interest detected and counted in the Day 14 biopsies are observed only rarely in unwounded skin. Therefore, they are comprised of neogenic-like and activated, stimulated, or reorganized structures. Table 8: Subject Demographics and Characteristics
Table 10: Photographic hair count of all hair on Day 168 in target analysis area subjected to dermabrasion plus hydrogel treatment
Table 11: Photographic hair count of non-vellus hair on Day 84 in target analysis area subjected to dermabrasion plus hydrogel treatment
it keeps going for a bit. Just go down and read it from the source.
https://www.google.com/patents/WO20...ved=0ahUKEwjR_LP1p-nNAhVFeD4KHWzaB6EQ6AEISTAG