alscarmuzza
Established Member
- Reaction score
- 127
Introduction: Finasteride (finasteride) is known as type II 5α-reductase inhibitor, which has been approved for the treatment and prevention of androgenetic alopecia. Administration of finasteride by oral route has led to undesirable systemic side effects that include mood disturbance, gynecomastia, decreased libido, erectile dysfunction, and ejaculation disorder. The aim was to improve finasteride delivery through skin layers and hair follicles that could possibly reduce its major side effects resulting from long-term oral administration for the treatment and prevention of male pattern baldness.
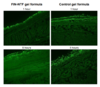
Materials and methods: finasteride was formulated as nano-transferosomal (NTF) gel formulations (F1–3). The prepared formulations were characterized for encapsulation efficiency, particle size, ex vivo skin permeation, and kinetic modeling. In addition, visualization of NTF skin penetration using a fluorescence laser microscope was carried out for the selected formula (F2).
Results and discussion: The results showed that finasteride encapsulation efficiency percentage was 69.72 ± 8.36, 89.43 ± 6.82, and 93.1 ± 1.93 for F1, F2, and F3, respectively. finasteride-NTF average vesicle sizes were 299.6 ± 45.6, 171 ± 25.6, and 197.4 ± 29.1 nm for F1, F2, and F3, respectively. finasteride-NTF formulations (F1–3) showed enhancement and improvement in the amount of finasteride permeated compared with raw finasteride gel formula. The NTF formula revealed uniform fluorescence (rhodamine) intensity across rat skin, which indicated improved delivery through skin layers compared with control gel formula.
Conclusion: These results indicated that NTF gel formula showed the ability to boost finasteride delivery across skin layers and could be applied as an alternative for oral therapy.
Materials and methods: finasteride was formulated as nano-transferosomal (NTF) gel formulations (F1–3). The prepared formulations were characterized for encapsulation efficiency, particle size, ex vivo skin permeation, and kinetic modeling. In addition, visualization of NTF skin penetration using a fluorescence laser microscope was carried out for the selected formula (F2).
Results and discussion: The results showed that finasteride encapsulation efficiency percentage was 69.72 ± 8.36, 89.43 ± 6.82, and 93.1 ± 1.93 for F1, F2, and F3, respectively. finasteride-NTF average vesicle sizes were 299.6 ± 45.6, 171 ± 25.6, and 197.4 ± 29.1 nm for F1, F2, and F3, respectively. finasteride-NTF formulations (F1–3) showed enhancement and improvement in the amount of finasteride permeated compared with raw finasteride gel formula. The NTF formula revealed uniform fluorescence (rhodamine) intensity across rat skin, which indicated improved delivery through skin layers compared with control gel formula.
Conclusion: These results indicated that NTF gel formula showed the ability to boost finasteride delivery across skin layers and could be applied as an alternative for oral therapy.