This paper (Harshuk-Shabso, S., Dressler, H., Niehrs, C. et al.) is a major breakthrough in understanding the onset of catagen, and how to delay it. We may even be able to put this information to practical use right now. The key revelation is that a drop in R-spondins is responsible for the transition from anagen to catagen, and that this reduction in R-spondins is mediated primarily by FGFR1 and FGFR2. When you silence FGFR1/2 then R-spondin levels remain elevated longer, and the anagen phase is extended for as long as R-spondins remain elevated. Eventually R-spondins still drop, and catagen is induced, but it's substantially delayed.
Rspo2/3 is a major factor in Androgenetic Alopecia pathology. They are mostly redundant, one or the other is all you need. See this excellent thread for a detailed explanation of the role of R-spondins in HF physiology. I will share a graph from that thread toward the end of this post.
From this new study:
Wnt signaling in the DP induces Rspo in the matrix, which creates a positive feedback loop allowing for b-catenin accumulation. Fgf signaling through FGFR1/2 increases throughout anagen, reducing Rspo levels, which interrupts the positive feedback loop and initiates catagen. If we silence FGFR1/2 we can use Wnt agonists to upregulate Rspo2/3 and keep hair in anagen.
Here is what happens in mice when you keep Wnt signaling high in the DP and silence FGFR1/2, delaying the reduction of R-spondins and the onset of catagen.
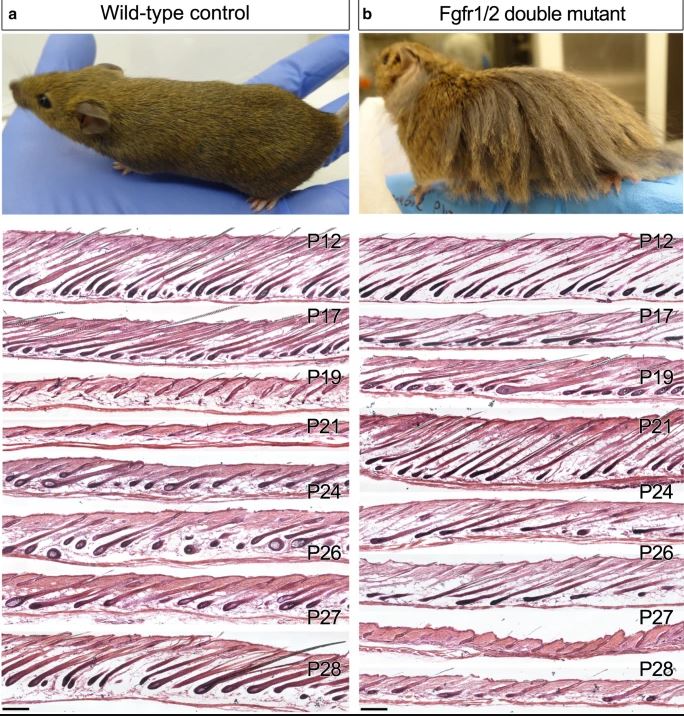

There have been studies over the last few years showing the importance of Rspo in treating Androgenetic Alopecia, and I believe a couple companies have been working on developing some Rspo2 agonists, but there's been no word on them. Perhaps you could pretty much cure Androgenetic Alopecia if you silence the AR(dutasteride/RU/CB), upregulate Wnts(GSK3b,SFRP1/2,DKK1/2 inhibition), and upregulate Rspo2/3.
The new cancer drug Infigratinib antagonizes FGFR. It is highly selective for FGFR1, FGFR2, and FGFR3, with a 40-60 fold affinity over FGFR4, and >128 fold over VEGFR2. It is not easily absorbed, so microneedling or DMSO will probably be required. It is an oral medication, but it is metabolized by CYP3A4 so it should be active topically. Due to CYP3A4 metabolism, ketoconazole and spironolactone are among the contraindicated drugs. Day 1 half-life is 3-7 hours, but plasma clearance is high, so oral dosing is 4x/day at 75-125mg, with 3 weeks on and 1 week off. Twice daily dosing should be sufficient for topical application, with the high plasma clearance reducing the potential for systemic side effects. The dose will be much lower topically than orally. As for the safety, there's no telling. It's currently undergoing clinical trials, but this link has some safety data from a phase II trial. I wouldn't want to use it orally.
Given this, hopefully less than 20mg/day topically will not cause side effects. I would think that dose would be more than sufficient with light skin disruption.
Infigratinib increased the length of hair/fur in rats and dogs over 13 weeks. That is great because it shows the murine study's findings are likely consistent with at least one larger animal model, increasing the possibility that it extends to humans.
This is the concerning part:
However, that is an oral dose that is probably 20-100x higher than what would be applied to the scalp.
Hair loss is actually a reported side effect of this drug(38%), but the patients in the clinical trials are not realizing the anagen promoting effect of silencing FGFR, because this only works in conjunction with Wnt signaling. Cancer patients would not be taking a Wnt agonist, they would be taking Wnt antagonists if anything. Hair loss would be expected in that case. However, when combined with a Wnt agonist hair growth would be expected. In my opinion the benefit of Rspo2/3 expression would substantially outweigh any downside to blocking fgf signaling in the DP as far as hair growth is concerned. I don't recommend using this drug, but it would certainly be interesting to see what it could do for Androgenetic Alopecia when combined with something like SM04554 or CHIR99021, or even minoxidil.
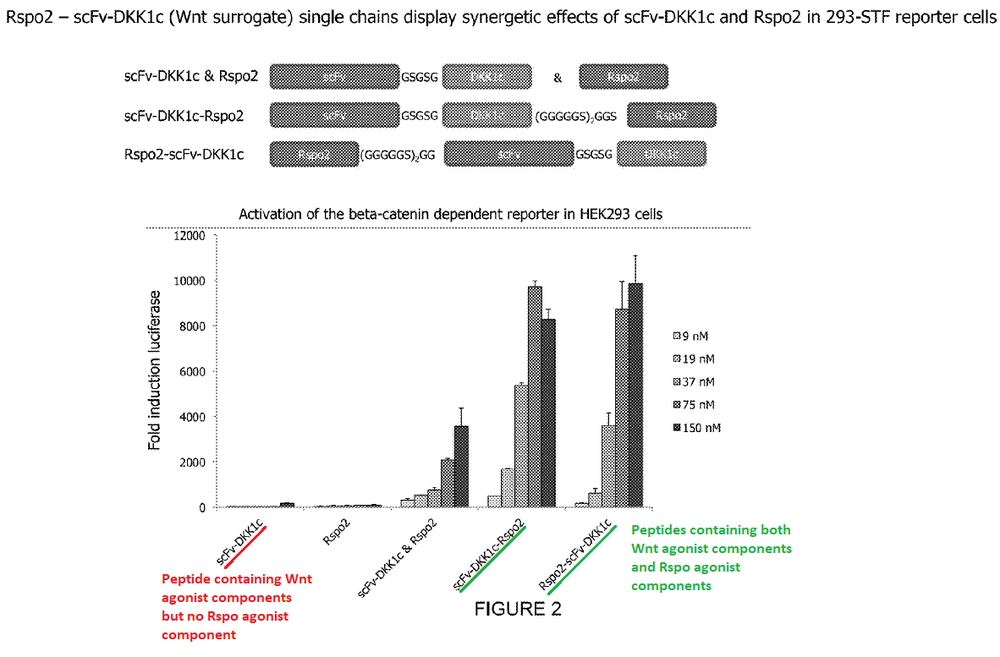
Here you can clearly see why hair growth would not be expected in the cancer trials with this drug. At least in kidney cells Rspo2 alone does little to induce beta-catenin. However, when combined with Wnt agonists, you can see the activation of beta-catenin is remarkable to say the least. The same appears to apply in the HF. Also, notice how the combination of Rspo2+Wnt agonists trounces Wnt agonists alone in terms of beta-catenin activation. Imagine the far left of the graph representing the hair growth potential of SM04554, while the right represents the potential of SM04554+Rspo2.
The only questions in my mind are: Are the available Wnt agonists sufficient for inducing beta-catenin and Rspo, or do we also need something like KY19382? Considering Samumed got decent results with their Wnt agonist in an Androgenetic Alopecia trial, I'd say that what we have is sufficient. So really the only question is, does silencing FGFR1/2 in a bald scalp prevent a drop in Rspo like it does in mice, and apparently dogs? I don't see why it wouldn't. If it does then this has a lot of potential. It's a shame we can't get our hands on Rspo2 recombinant protein. We could keep hair in anagen forever.
Fgf signaling in the DP modulates this positive loop by progressively suppressing the expression of Rspondins (Fig. 8). The kinetics by which this reduction occurs define the period required to reduce Wnt activity in the matrix and to promote the induction of the regression phase, and consequently, control the duration of anagen. Remarkably, this decline in expression of Rspondins in wild-type mice starts ~4 days before the appearance of any morphological signs of catagen induction.
Rspo2/3 is a major factor in Androgenetic Alopecia pathology. They are mostly redundant, one or the other is all you need. See this excellent thread for a detailed explanation of the role of R-spondins in HF physiology. I will share a graph from that thread toward the end of this post.
From this new study:
This is consistent with Rspondins being target genes of Wnt signaling and with the dramatic reduction in Wnt activity in the matrix when beta-catenin is ablated in the DP. Furthermore, this suggests that the extended anagen, observed when Fgfr1 and Fgfr2 were ablated in the DP, requires the presence of active Wnt signaling in the DP.
Wnt signaling in the DP induces Rspo in the matrix, which creates a positive feedback loop allowing for b-catenin accumulation. Fgf signaling through FGFR1/2 increases throughout anagen, reducing Rspo levels, which interrupts the positive feedback loop and initiates catagen. If we silence FGFR1/2 we can use Wnt agonists to upregulate Rspo2/3 and keep hair in anagen.
The absence of Fgf signaling in the DP of the dMF1/2 mutant at P16 prevents the elevation of both Dkk2 and Notum, suggesting that Dkk2 and Notum are transcriptionally activated by Fgf signaling in the DP...
Furthermore, together these data support that (1) while both Dkk2 and Notum may participate in regulating the initiation of catagen, they are not required to induce catagen; and (2) the reduction of Rspondin levels during mid-to-late anagen is the preponderant driving force that promotes and induces catagen.
Here is what happens in mice when you keep Wnt signaling high in the DP and silence FGFR1/2, delaying the reduction of R-spondins and the onset of catagen.

Note that suppression of Rspondins by Fgf signaling in the DP occurs in the presence of active Wnt signaling in DP cells (Figs. 3d and 4b–d, Supplementary Fig. 5a), suggesting that Fgf signaling downregulates the expression of Rspondins downstream to beta-catenin activation. This is in line with a previous report illustrating that forced expression of the constitutive active form of beta-catenin specifically in the DP does not alter the hair cycle27. Mechanistically, Fgf signaling in the DP may antagonize beta-catenin activity in the DP at the promoter level of Rspondins, predicting a wide range of promotor activity that depends on the particular promoter. This is corroborated by the observation that ablation of beta-catenin in the DP results in complete abolishment of all Rspondins at the transcript level (Fig. 6d–g), while abrogation of Fgf signaling in the DP preferentially affects the expression of Rspo2 and Rspo3
It is noteworthy that while ablation of Fgfr1 and Fgfr2 in the DP results in anagen extension, mutant follicles eventually enter catagen. Furthermore, the reduction in Rspondins expression in the DP of the Fgfr1/2 double mutant does occur during the extended anagen but apparently at a slower pace, corroborating that catagen induction at the molecular level occurs long before the actual regression of the follicle and suggesting that additional molecular components or pathways are involved in regulating the same molecular pacemaker. While elevation of Fgfr3 and Fgfr4 in the DP of the dMF1/2 mutant during the extended anagen to compensate for the loss of Fgfr1 and Fgfr2 was not observed, the very low levels of Fgfr3 and Fgfr4 may be sufficient for contributing to the regulation of the hair cycle clock in the DP but require a longer time to suppress the expression of Rspondins. Alternatively, other receptor tyrosine kinases are also involved in regulating the hair cycle clock in the DP through cooperative activation of the same intracellular signal transduction pathways activated by Fgfr, and ablation of Fgfr receptors in the DP reduces the activation levels of these pathways but does not abolish them completely.
While the current study did not aim to address the regulation of Fgf signaling in the DP upstream to the Fgf receptors, previous works suggest that Fgf5 is likely to contribute to this regulation. Fgf5 is expressed in the lower ORS and lower matrix21. Furthermore, Fgf5 knockout mice exhibit extended anagen. Combined with our analysis, these data together suggest that Fgf5 acts on DP cells to regulate the duration of anagen. Alternatively, Fgf5 function is relayed to the DP through activation of other Fgf ligands in the matrix that trigger Fgf transduction in the DP. However, anagen in the Fgf5 knockout mice is extended by 3 days22 while ablation of Fgfr1 and Fgfr2 in the DP results in anagen extension between 6 and 8 days. This suggests that a combined action of a few Fgf ligands is involved.
There have been studies over the last few years showing the importance of Rspo in treating Androgenetic Alopecia, and I believe a couple companies have been working on developing some Rspo2 agonists, but there's been no word on them. Perhaps you could pretty much cure Androgenetic Alopecia if you silence the AR(dutasteride/RU/CB), upregulate Wnts(GSK3b,SFRP1/2,DKK1/2 inhibition), and upregulate Rspo2/3.
The new cancer drug Infigratinib antagonizes FGFR. It is highly selective for FGFR1, FGFR2, and FGFR3, with a 40-60 fold affinity over FGFR4, and >128 fold over VEGFR2. It is not easily absorbed, so microneedling or DMSO will probably be required. It is an oral medication, but it is metabolized by CYP3A4 so it should be active topically. Due to CYP3A4 metabolism, ketoconazole and spironolactone are among the contraindicated drugs. Day 1 half-life is 3-7 hours, but plasma clearance is high, so oral dosing is 4x/day at 75-125mg, with 3 weeks on and 1 week off. Twice daily dosing should be sufficient for topical application, with the high plasma clearance reducing the potential for systemic side effects. The dose will be much lower topically than orally. As for the safety, there's no telling. It's currently undergoing clinical trials, but this link has some safety data from a phase II trial. I wouldn't want to use it orally.
At 5 and 10 mg/day, plasma concentrations were low and frequently below the lower limit of quantification. Plasma concentrations were consistently quantifiable starting at 20 mg/day
Given this, hopefully less than 20mg/day topically will not cause side effects. I would think that dose would be more than sufficient with light skin disruption.
Infigratinib increased the length of hair/fur in rats and dogs over 13 weeks. That is great because it shows the murine study's findings are likely consistent with at least one larger animal model, increasing the possibility that it extends to humans.
In the 13-week rat toxicity study, besides the cornea thinning, additional effects on the epithelium (thinning or degeneration) were reported mainly for the tongue, nasal cavity and incisors (non-growing teeth such as the molar teeth were not affected). In the 13-week dog toxicity study only, atrophy of the meibomian and sebaceous gland was reported. In rat and dog 13-week toxicity study, a change in the coat appearance (denoted by relatively longer hair/fur) was noted. This finding did not show any microscopic correlate.
This is the concerning part:
In repeated dose (oral gavage; up to 4-weeks) toxicity studies, BGJ398 (infigratinib) did lead to increases in serum FGF23 and serum phosphorous associated with partially reversible ectopic mineralization (kidney, lung, vascular and digestive systems) along with largely reversible changes in renal function parameters and bone growth plate thickening / retention of the primary spongiosa in rats (≥ 10 mg/kg/day) and dogs (≥ 10 mg/kg/day).
However, that is an oral dose that is probably 20-100x higher than what would be applied to the scalp.
Hair loss is actually a reported side effect of this drug(38%), but the patients in the clinical trials are not realizing the anagen promoting effect of silencing FGFR, because this only works in conjunction with Wnt signaling. Cancer patients would not be taking a Wnt agonist, they would be taking Wnt antagonists if anything. Hair loss would be expected in that case. However, when combined with a Wnt agonist hair growth would be expected. In my opinion the benefit of Rspo2/3 expression would substantially outweigh any downside to blocking fgf signaling in the DP as far as hair growth is concerned. I don't recommend using this drug, but it would certainly be interesting to see what it could do for Androgenetic Alopecia when combined with something like SM04554 or CHIR99021, or even minoxidil.
Here you can clearly see why hair growth would not be expected in the cancer trials with this drug. At least in kidney cells Rspo2 alone does little to induce beta-catenin. However, when combined with Wnt agonists, you can see the activation of beta-catenin is remarkable to say the least. The same appears to apply in the HF. Also, notice how the combination of Rspo2+Wnt agonists trounces Wnt agonists alone in terms of beta-catenin activation. Imagine the far left of the graph representing the hair growth potential of SM04554, while the right represents the potential of SM04554+Rspo2.
The only questions in my mind are: Are the available Wnt agonists sufficient for inducing beta-catenin and Rspo, or do we also need something like KY19382? Considering Samumed got decent results with their Wnt agonist in an Androgenetic Alopecia trial, I'd say that what we have is sufficient. So really the only question is, does silencing FGFR1/2 in a bald scalp prevent a drop in Rspo like it does in mice, and apparently dogs? I don't see why it wouldn't. If it does then this has a lot of potential. It's a shame we can't get our hands on Rspo2 recombinant protein. We could keep hair in anagen forever.
Last edited:
