- Reaction score
- 550
According to this old thread:
I will give some insights to Alfatradiol and 3a-Diol-G.
Firstly, the initial study posted by @parisienne is the famous study about the aromatization that 17a-Estradiol (Alfatradiol) provides. So there is a misconception regarding 17a and 17b-Estradiol. Check it here: https://pubmed.ncbi.nlm.nih.gov/12190948/
"Furthermore, we show that in comparison to the controls, we noticed in 17alpha-estradiol-incubated (1 nM) female hair follicles a concentration- and time-dependent increase of aromatase activity (at 24 h: 1 nM = +18%, 100 nM = +25%, 1 micro M = +57%; 24 h: 1 nM = +18%, 48 h: 1 nM = +25%). In conclusion, our ex vivo experiments suggest that under the influence of 17alpha-estradiol an increased conversion of testosterone to 17beta-estradiol and androstendione to estrone takes place, which might explain the beneficial effects of estrogen treatment of Androgenetic Alopecia."
What I posted a few months back:
In my opinion, Alfatradiol seems to be underrated. Maybe the 0.025% it's not very much or maybe is enough for others, but with higher doses, someone might achieve better results. Forget it to act as a DHT blocker when someone is already on Finasteride or Dutasteride because the latter will do the job, outperforming Alfatradiol in the 5AR blockade. The interesting part of Alfatradiol is the increment in Aromatase Activity and the capability to block 17β-HSD. We know that Aromatase is so important for Hair Follicles and I believe that's the most interesting part of Alfatradiol. Furthermore, as much as it can block the 17β-HSD it's a plus as well because is blocking the conversion of T to Androstenedione, thus promoting the conversion to the Estrogens, Estrone, and Estradiol. The only problem is the ERα receptor binding affinity (or maybe is not?)
1)https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3412238/ (Mechanism)
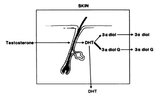
In the treatment of androgenetic alopecia, the action mechanism of 17α-estradiol is to suppress 5α-reductase activity, which impedes the conversion of testosterone to the more potent metabolite DHT. In addition, it inhibits 17β-dehydrogenase activity, resulting in a slowing of the conversion process of androstenedione to testosterone. As a result, there is a reduction in the synthesis of testosterone and DHT. On the other hand, by stimulating aromatase, the conversion of testosterone to estradiol is accelerated, hence, testosterone is reduced. It thus acts to ultimately reduce DHT. In addition, it has been reported to accelerate the generation of hair follicular matrix cells.
2)https://pubmed.ncbi.nlm.nih.gov/9284093/ (Aromatase/5AR levels on Scalp)
Findings revealed that both women and men have higher levels of receptors and 5alpha-reductase type I and II in frontal hair follicles than in occipital follicles, whereas higher levels of aromatase were found in their occipital follicles. There are marked quantitative differences in levels of androgen receptors and the three enzymes, which we find to be primarily in the outer root sheath of the hair follicles in the two genders. Androgen receptor content in female frontal hair follicles was approximately 40% lower than in male frontal hair follicles. Cytochrome P-450-aromatase content in women's frontal hair follicles was six times greater than in frontal hair follicles in men. Frontal hair follicles in women had 3 and 3.5 times less 5alpha-reductase type I and II, respectively than frontal hair follicles in men. These differences in levels of androgen receptor and steroid-converting enzymes may account for the different clinical presentations of androgenetic alopecia in women and men.
3)https://pubmed.ncbi.nlm.nih.gov/12190948/ (17a-Estradiol Induces Aromatase Activity)
For the topical treatment of androgenetic alopecia (Androgenetic Alopecia) in women, solutions containing either estradiol benzoate, estradiol valerate, 17beta- or 17alpha-estradiol are commercially available in Europe and some studies show an increased anagen and decreased telogen rate after treatment as compared with placebo. At present, it is not precisely known how estrogens mediate their beneficial effect on Androgenetic Alopecia-affected hair follicles. We have shown recently that 17alpha-estradiol is able to diminish the amount of dihydrotestosterone (DHT) formed by human hair follicles after incubation with testosterone while increasing the concentration of weaker steroids such as estrogens. Because aromatase is involved in the conversion of testosterone to estrogens and because there is some clinical evidence that aromatase activity may be involved in the pathogenesis of Androgenetic Alopecia, we addressed the question of whether aromatase is expressed in human hair follicles and whether 17alpha-estradiol is able to modify the aromatase activity. Herewith we were able to demonstrate that intact, microdissected hair follicles from female donors express considerably more aromatase activity than hair follicles from male donors. Using immunohistochemistry, we detected the aromatase mainly in the epithelial parts of the hair follicle and not in the dermal papilla. Furthermore, we show that in comparison to the controls, we noticed in 17alpha-estradiol-incubated (1 nM) female hair follicles a concentration- and time-dependent increase of aromatase activity (at 24 h: 1 nM = +18%, 100 nM = +25%, 1 micro M = +57%; 24 h: 1 nM = +18%, 48 h: 1 nM = +25%). In conclusion, our ex vivo experiments suggest that under the influence of 17alpha-estradiol an increased conversion of testosterone to 17beta-estradiol and androstenedione to estrone takes place, which might explain the beneficial effects of estrogen treatment of Androgenetic Alopecia.
4) Affinities:
Other investigators have found diverse affinities, as well: Kuiper et al. (1997) [44] reported an affinity of 17 α-E2 to ERα of 58% of the relative affinity of 17 β-E2, and 11% to ERβ, while Torand-Allerand et al. (2005) [26] reported an affinity of 17 α-E2 bindings to human recombinant ERα and ERβ of 51 and 64% compared to 17 β-E2, respectively. Kaur et al. (2015) [45] indicated an affinity of 17 α-E2 to ERα to be 40-times lower than 17 β-E2.”
5)General Information for 17a-Estradiol (Recent):
6) Comparison with Topical Finasteride: (But the thing here is: What if you combine them? Different Mechanisms and you need to yield every possible positive effect)
Efficacy of Topical Finasteride 0.5% vs 17α-Estradiol 0.05% in the Treatment of Postmenopausal Female Pattern Hair Loss: A Retrospective, Single-Blind Study of 119 Patients
From wiki:
"3α-Hydroxysteroid dehydrogenase (3α-HSD or aldo-keto reductase family 1 member C4) is an enzyme that in humans is encoded by the AKR1C4 gene. It is known to be necessary for the synthesis of the endogenous neurosteroids allopregnanolone, THDOC, and 3α-androstanediol. It is also known to catalyze the reversible conversion of 3α-androstanediol (5α-androstane-3α,17β-diol) to dihydrotestosterone (DHT, 5α-androstan-17β-ol-3-one) and vice versa."
I can't tell much, but I will stand to 3a-Diol-G.
"3α-Androstanediol glucuronide (3α-ADG) is a metabolite formed from human androgens; compounds involved in the development and maintenance of sexual characteristics. It is formed by the glucuronidation of both dihydrotestosterone and testosterone, and has been proposed as means of measuring androgenic activity.
In women the adrenal steroids, dehydroepiandrosterone sulfate, androstenedione, and dehydroepiandrosterone are the major precursors of plasma 3α-ADG, accounting for almost the totality of circulating 3α-ADG. Levels of 3α-ADG decrease significantly with age.
3α-ADG is used as a marker of target tissue cellular action. 3α-ADG correlates with level of 5α-reductase activity (testosterone and 3α-androstanediol to dihydrotestosterone) in the skin. Concentrations of 3α-ADG are associated with the level of cutaneous androgen metabolism."
I kinda experience it with my 3a-Diol-G levels, which is a major metabolite of DHT. When these levels were low, my hair felt better and in general, the androgen activity on the scalp is lower(possibly). At least they are correlating:
"Several factors may be involved in the pathogenesis of female baldness. Androgens originating from the adrenal gland (eg, DHEA), ovary (eg, DHEA and testosterone), and hair follicle cells (eg, DHT and 3a-diol G) have been implicated. Plasma levels of DHEA and its sulfate decline with age, as do those of androstenedione, while testosterone and free testosterone levels remain about the same regardless of age ion rp^g serum levels of DHT, testosterone, 3a-diol G, and SHBG correlate with the peripheral metabolic activity of the target organs on the precursor hormones.10" All scalp hairs are exposed to the same androgen concentrations, but the balding follicles are genetically susceptible to the androgen factor. The androgen factor and genetic factor are thought to operate through a central system, possibly the cyclic adenosine monophosphate protein kinase system, to affect the metamorphosis of the terminal into the vellus-type follicle. Its effects on the proliferation and metabolism of the matrix cell of the hair follicle may be central to the balding process. The peripheral (ie, extraglandular) conversion of androgens is particularly important in women since it is the major route by which testosterone is formed. The conversion of testosterone and DHEA to DHT and the androstanediols occurs in the skin through the 5a-reductase and 17/3-hydroxysteroid dehydroge¬ nase systems.1516 Studies on isolated plucked scalp hairs have demonstrated that testosterone metabolism in both the nonbalding man and woman occurs primarily through the 17/î-hydroxysteroid dehydrogenase system to androstenedione and secondarily through the 5«-reductase system to dihydrotestosterone. In balding men, the rate of 5a-reductase activity is increased, leading to increased concentrations of dihydrotestosterone.18 The major metabolites of dihydrotestosterone, when incubated with skin and skin structures from the scalp, are 3a-diol G and 3/3-diol G. The increased activity of dihydrotestosterone in the balding scalp may be reflected in slightly increased concentrations of 3a-diol G in the tissue and plasma. The sex hormone binding protein is a /3-globulin, which is synthesized in the liver. The binding of androgens to this transport protein is physiologically and pathologically important since it appears that it is the proportion of the androgen not bound to SHBG that is biologically active. Decreased production of SHBG has been reported in hirsutism, polycystic ovarian disease, exogenous androgens, and syndromes of androgen excess. The decreased SHBG provides a better index for hyperandrogenicity than do either the total or free testosterone levels. Changes in the concentration of binding protein alter the availability of androgens, which bind to the cells that affect androgen clearance. The higher clearance rate results from an increase in peripheral metabolism reflected in increased plasma levels and excretion of 5a-reduced metabolites such as 3a-diol G. The 3a-diol G levels are markedly elevated in the plasma of many patients with idiopathic hirsutism and have been used as a marker of peripheral androgen action. This study supports the concept of increased androgen production in some subjects with female pattern baldness, as reflected by the depressed SHBG level and slightly increased 3a-diol G level compared with those in both the control group of women and women with male pattern baldness. The marked increase of the 3a-diol G/SHBG ratio suggests that these women may be losing hair due to genetically sensitive hair bulbs responding to a small excess of androgen but without the maximal androgen expression of hirsutism, acne, or virilism. Our results did not confirm increased serum testosterone levels or an increased serum T/SHBG ratio in female patients with male pattern baldness, as had been suggested in previous reports. We also were not able to confirm previous findings of elevated DHEA sulfate levels in the subjects with female pattern baldness. The T/SHBG ratio was not a helpful marker for this type of hair loss. This study suggests that a low SHBG level and an increased ratio of 3a-diol G to SHBG are characteristic of the female pattern type of hair loss in young women. It is interesting to speculate that decreased SHBG concentrations result in increased availability of androgens for uptake and subsequent metabolism in the hair follicle in this group of women. Further study is needed to clarify the role of androgens and their relationship to the genetic factors that determine the effects of androgen metabolism in susceptible hair follicles in balding women."
Information is gathered exclusively from this study:https://pubmed.ncbi.nlm.nih.gov/2943232/
So, when I checked my 3a-Diol-G levels again a few months back, they were almost in the high-end levels, and my hair quality was worse. Probably because of the higher androgen activity on the scalp? (Could it be)
(August 2019: Started Topical Finasteride:Baseline levels of DHT: 541 pg/ml
Otober 2019: DHT levels:302 pg/ml
January 2020: DHT levels:298 pg/ml-After starting Oral Finasteride as well ( I was already on it for about 3 weeks-0.5mg 3 times/week)
May 2020: DHT levels:313 pg/ml-I jumped into 1mg daily from 31st of March. I jumped to 0.5mg everyday from mid February
June 2020: DHT levels:283 pg/ml- I even tested 3a-Diol-G which is far a strongest indicator than DHT alone. 3a-Diol-G is a major DHT metabolite and it can converts back to DHT as well. It came low at 1.3 ng/ml while the range is: 3.4-22.
September 2020: DHT levels: 383 pg/ml
January 2021: DHT levels: 550 pg/ml !!! Back to baseline!-I checked as well my PSA levels and it was 0.23 ng/ml but I don't have baseline levels unfortunately.
May 2021 (Latest): I skipped DHT this time and tested only 3a-Diol-G. I got a result of: 16.1 this time!!! It makes sense, doesn't it? Higher DHT->Higher metabolite->Higher Androgenicity overall. I assume that my DHT is still high!-----Upregulated 5AR+Upregulated potent Androgen metabolites (3a-diol-G), destructive combination I guess.)
Another study (males this time):
"Serum 3a-androstanediol glucuronide (3a-Adiol-G) is considered to be an indicator of peripheral tissue androgen metabolism. Precursor circulating androgens are converted in peripheral tissue to dihydrotestosterone (DHT), which is ultimately metabolized to 3a-Adiol-G and secreted from the cell. Elevated serum 3a-Adiol-G concentrations have been reported in women in hyperandrogenic states. We studied 44 consecutive male medical students for chest hair density, acne, and serum dehydroepiandrosterone sulfate (DHEA-S), total testosterone (total T), free and albumin-bound (bioavailable) T (bio T), and 3a-Adiol-G concentrations. Although there was a considerable overlap of serum 3a-Adiol-G values among the groups defined by hair density or acne scores, we found statistically significant correlations between serum 3a-Adiol-G and chest hairiness (P = 0.0034), acne (P = 0.0005), and a combined chest hairiness and acne score (P = 0.0018). There was no significant correlation between these clinical parameters and the levels of precursor androgens. There was, however, a strong correlation between serum 3a-Adiol-G and bio T (P = 0.0005), suggesting that in men serum 3a-Adiol-G levels may be dependent upon available free and albumin-bound T. The correlations in men of serum 3a-Adiol-G with chest hair density, acne, and the hairiness and acne index supports the hypothesis that the serum levels of 3a-Adiol-G reflect the extent of androgen action in peripheral tissues."
Study:https://pubmed.ncbi.nlm.nih.gov/2972739/
As for the estrogen receptor alpha, I can't provide much information because it is somewhat complex. I will provide though one of the most detailed studies about Estrogens on hair follicles: https://academic.oup.com/edrv/article/27/6/677/2355194 (I will stand that the effects seem to be HIGHLY gender-sex specific and different when it comes to animals and humans. At least this is my understanding. Also, maybe Alfatradiol potency is not great for the ERα effects, since it is not as strong as 17b-Estradiol. Like @pariesienne said above, 17b inhibited hair growth while 17a not. and this is mentioned in the above study as well: Oh and Smart found that, in mice, topical E2 administration to clipped dorsal skin arrested hair follicles in telogen and produced a profound and prolonged inhibition of hair growth, whereas treatment with the biologically inactive stereoisomer 17α-estradiol did not alter hair growth.)
I will include as well a translated german study for Alfatradiol. Is one of the most detailed because there are males as well.
*Link for the 2005 study: https://www.thieme-connect.de/products/ejournals/html/10.1055/s-2005-870188
*Use a pdf translator.
TL;DR: Aromatase is very important for hair follicles, as we know. Estrogen Receptors Alpha and Beta are still kind of complex for me to explain them better and more detailed. 3a-Diol-G is probably a great indicator for androgen activity in tissues, including probably the scalp as well. Maybe it's worth it for someone to give at least a try to Alfatradiol, as it should stop testosterone and androstenedione as well to some extent, but not to Alfatradiol alone. Combination therapy is always the point. You have to connect as many dots as you can with all the mechanisms available.
Thanks for reading and sorry for any mistakes.
I will give some insights to Alfatradiol and 3a-Diol-G.
Firstly, the initial study posted by @parisienne is the famous study about the aromatization that 17a-Estradiol (Alfatradiol) provides. So there is a misconception regarding 17a and 17b-Estradiol. Check it here: https://pubmed.ncbi.nlm.nih.gov/12190948/
"Furthermore, we show that in comparison to the controls, we noticed in 17alpha-estradiol-incubated (1 nM) female hair follicles a concentration- and time-dependent increase of aromatase activity (at 24 h: 1 nM = +18%, 100 nM = +25%, 1 micro M = +57%; 24 h: 1 nM = +18%, 48 h: 1 nM = +25%). In conclusion, our ex vivo experiments suggest that under the influence of 17alpha-estradiol an increased conversion of testosterone to 17beta-estradiol and androstendione to estrone takes place, which might explain the beneficial effects of estrogen treatment of Androgenetic Alopecia."
What I posted a few months back:
In my opinion, Alfatradiol seems to be underrated. Maybe the 0.025% it's not very much or maybe is enough for others, but with higher doses, someone might achieve better results. Forget it to act as a DHT blocker when someone is already on Finasteride or Dutasteride because the latter will do the job, outperforming Alfatradiol in the 5AR blockade. The interesting part of Alfatradiol is the increment in Aromatase Activity and the capability to block 17β-HSD. We know that Aromatase is so important for Hair Follicles and I believe that's the most interesting part of Alfatradiol. Furthermore, as much as it can block the 17β-HSD it's a plus as well because is blocking the conversion of T to Androstenedione, thus promoting the conversion to the Estrogens, Estrone, and Estradiol. The only problem is the ERα receptor binding affinity (or maybe is not?)
1)https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3412238/ (Mechanism)
In the treatment of androgenetic alopecia, the action mechanism of 17α-estradiol is to suppress 5α-reductase activity, which impedes the conversion of testosterone to the more potent metabolite DHT. In addition, it inhibits 17β-dehydrogenase activity, resulting in a slowing of the conversion process of androstenedione to testosterone. As a result, there is a reduction in the synthesis of testosterone and DHT. On the other hand, by stimulating aromatase, the conversion of testosterone to estradiol is accelerated, hence, testosterone is reduced. It thus acts to ultimately reduce DHT. In addition, it has been reported to accelerate the generation of hair follicular matrix cells.
2)https://pubmed.ncbi.nlm.nih.gov/9284093/ (Aromatase/5AR levels on Scalp)
Findings revealed that both women and men have higher levels of receptors and 5alpha-reductase type I and II in frontal hair follicles than in occipital follicles, whereas higher levels of aromatase were found in their occipital follicles. There are marked quantitative differences in levels of androgen receptors and the three enzymes, which we find to be primarily in the outer root sheath of the hair follicles in the two genders. Androgen receptor content in female frontal hair follicles was approximately 40% lower than in male frontal hair follicles. Cytochrome P-450-aromatase content in women's frontal hair follicles was six times greater than in frontal hair follicles in men. Frontal hair follicles in women had 3 and 3.5 times less 5alpha-reductase type I and II, respectively than frontal hair follicles in men. These differences in levels of androgen receptor and steroid-converting enzymes may account for the different clinical presentations of androgenetic alopecia in women and men.
3)https://pubmed.ncbi.nlm.nih.gov/12190948/ (17a-Estradiol Induces Aromatase Activity)
For the topical treatment of androgenetic alopecia (Androgenetic Alopecia) in women, solutions containing either estradiol benzoate, estradiol valerate, 17beta- or 17alpha-estradiol are commercially available in Europe and some studies show an increased anagen and decreased telogen rate after treatment as compared with placebo. At present, it is not precisely known how estrogens mediate their beneficial effect on Androgenetic Alopecia-affected hair follicles. We have shown recently that 17alpha-estradiol is able to diminish the amount of dihydrotestosterone (DHT) formed by human hair follicles after incubation with testosterone while increasing the concentration of weaker steroids such as estrogens. Because aromatase is involved in the conversion of testosterone to estrogens and because there is some clinical evidence that aromatase activity may be involved in the pathogenesis of Androgenetic Alopecia, we addressed the question of whether aromatase is expressed in human hair follicles and whether 17alpha-estradiol is able to modify the aromatase activity. Herewith we were able to demonstrate that intact, microdissected hair follicles from female donors express considerably more aromatase activity than hair follicles from male donors. Using immunohistochemistry, we detected the aromatase mainly in the epithelial parts of the hair follicle and not in the dermal papilla. Furthermore, we show that in comparison to the controls, we noticed in 17alpha-estradiol-incubated (1 nM) female hair follicles a concentration- and time-dependent increase of aromatase activity (at 24 h: 1 nM = +18%, 100 nM = +25%, 1 micro M = +57%; 24 h: 1 nM = +18%, 48 h: 1 nM = +25%). In conclusion, our ex vivo experiments suggest that under the influence of 17alpha-estradiol an increased conversion of testosterone to 17beta-estradiol and androstenedione to estrone takes place, which might explain the beneficial effects of estrogen treatment of Androgenetic Alopecia.
4) Affinities:
Other investigators have found diverse affinities, as well: Kuiper et al. (1997) [44] reported an affinity of 17 α-E2 to ERα of 58% of the relative affinity of 17 β-E2, and 11% to ERβ, while Torand-Allerand et al. (2005) [26] reported an affinity of 17 α-E2 bindings to human recombinant ERα and ERβ of 51 and 64% compared to 17 β-E2, respectively. Kaur et al. (2015) [45] indicated an affinity of 17 α-E2 to ERα to be 40-times lower than 17 β-E2.”
5)General Information for 17a-Estradiol (Recent):
Health benefits attributed to 17α-estradiol, a lifespan-extending compound, are mediated through estrogen receptor α
https://www.biorxiv.org/content/10.1101/2020.06.02.130674v1.full (or here: https://pubmed.ncbi.nlm.nih.gov/33289482/)6) Comparison with Topical Finasteride: (But the thing here is: What if you combine them? Different Mechanisms and you need to yield every possible positive effect)
Efficacy of Topical Finasteride 0.5% vs 17α-Estradiol 0.05% in the Treatment of Postmenopausal Female Pattern Hair Loss: A Retrospective, Single-Blind Study of 119 Patients
Efficacy of Topical Finasteride 0.5% vs 17α-Estradiol 0.05% in the Treatment of Postmenopausal Female Pattern Hair Loss: A Retrospective, Single-Blind Study of 119 Patients.
Regarding now 3a-HSD:From wiki:
"3α-Hydroxysteroid dehydrogenase (3α-HSD or aldo-keto reductase family 1 member C4) is an enzyme that in humans is encoded by the AKR1C4 gene. It is known to be necessary for the synthesis of the endogenous neurosteroids allopregnanolone, THDOC, and 3α-androstanediol. It is also known to catalyze the reversible conversion of 3α-androstanediol (5α-androstane-3α,17β-diol) to dihydrotestosterone (DHT, 5α-androstan-17β-ol-3-one) and vice versa."
I can't tell much, but I will stand to 3a-Diol-G.
"3α-Androstanediol glucuronide (3α-ADG) is a metabolite formed from human androgens; compounds involved in the development and maintenance of sexual characteristics. It is formed by the glucuronidation of both dihydrotestosterone and testosterone, and has been proposed as means of measuring androgenic activity.
In women the adrenal steroids, dehydroepiandrosterone sulfate, androstenedione, and dehydroepiandrosterone are the major precursors of plasma 3α-ADG, accounting for almost the totality of circulating 3α-ADG. Levels of 3α-ADG decrease significantly with age.
3α-ADG is used as a marker of target tissue cellular action. 3α-ADG correlates with level of 5α-reductase activity (testosterone and 3α-androstanediol to dihydrotestosterone) in the skin. Concentrations of 3α-ADG are associated with the level of cutaneous androgen metabolism."
I kinda experience it with my 3a-Diol-G levels, which is a major metabolite of DHT. When these levels were low, my hair felt better and in general, the androgen activity on the scalp is lower(possibly). At least they are correlating:
"Several factors may be involved in the pathogenesis of female baldness. Androgens originating from the adrenal gland (eg, DHEA), ovary (eg, DHEA and testosterone), and hair follicle cells (eg, DHT and 3a-diol G) have been implicated. Plasma levels of DHEA and its sulfate decline with age, as do those of androstenedione, while testosterone and free testosterone levels remain about the same regardless of age ion rp^g serum levels of DHT, testosterone, 3a-diol G, and SHBG correlate with the peripheral metabolic activity of the target organs on the precursor hormones.10" All scalp hairs are exposed to the same androgen concentrations, but the balding follicles are genetically susceptible to the androgen factor. The androgen factor and genetic factor are thought to operate through a central system, possibly the cyclic adenosine monophosphate protein kinase system, to affect the metamorphosis of the terminal into the vellus-type follicle. Its effects on the proliferation and metabolism of the matrix cell of the hair follicle may be central to the balding process. The peripheral (ie, extraglandular) conversion of androgens is particularly important in women since it is the major route by which testosterone is formed. The conversion of testosterone and DHEA to DHT and the androstanediols occurs in the skin through the 5a-reductase and 17/3-hydroxysteroid dehydroge¬ nase systems.1516 Studies on isolated plucked scalp hairs have demonstrated that testosterone metabolism in both the nonbalding man and woman occurs primarily through the 17/î-hydroxysteroid dehydrogenase system to androstenedione and secondarily through the 5«-reductase system to dihydrotestosterone. In balding men, the rate of 5a-reductase activity is increased, leading to increased concentrations of dihydrotestosterone.18 The major metabolites of dihydrotestosterone, when incubated with skin and skin structures from the scalp, are 3a-diol G and 3/3-diol G. The increased activity of dihydrotestosterone in the balding scalp may be reflected in slightly increased concentrations of 3a-diol G in the tissue and plasma. The sex hormone binding protein is a /3-globulin, which is synthesized in the liver. The binding of androgens to this transport protein is physiologically and pathologically important since it appears that it is the proportion of the androgen not bound to SHBG that is biologically active. Decreased production of SHBG has been reported in hirsutism, polycystic ovarian disease, exogenous androgens, and syndromes of androgen excess. The decreased SHBG provides a better index for hyperandrogenicity than do either the total or free testosterone levels. Changes in the concentration of binding protein alter the availability of androgens, which bind to the cells that affect androgen clearance. The higher clearance rate results from an increase in peripheral metabolism reflected in increased plasma levels and excretion of 5a-reduced metabolites such as 3a-diol G. The 3a-diol G levels are markedly elevated in the plasma of many patients with idiopathic hirsutism and have been used as a marker of peripheral androgen action. This study supports the concept of increased androgen production in some subjects with female pattern baldness, as reflected by the depressed SHBG level and slightly increased 3a-diol G level compared with those in both the control group of women and women with male pattern baldness. The marked increase of the 3a-diol G/SHBG ratio suggests that these women may be losing hair due to genetically sensitive hair bulbs responding to a small excess of androgen but without the maximal androgen expression of hirsutism, acne, or virilism. Our results did not confirm increased serum testosterone levels or an increased serum T/SHBG ratio in female patients with male pattern baldness, as had been suggested in previous reports. We also were not able to confirm previous findings of elevated DHEA sulfate levels in the subjects with female pattern baldness. The T/SHBG ratio was not a helpful marker for this type of hair loss. This study suggests that a low SHBG level and an increased ratio of 3a-diol G to SHBG are characteristic of the female pattern type of hair loss in young women. It is interesting to speculate that decreased SHBG concentrations result in increased availability of androgens for uptake and subsequent metabolism in the hair follicle in this group of women. Further study is needed to clarify the role of androgens and their relationship to the genetic factors that determine the effects of androgen metabolism in susceptible hair follicles in balding women."
Information is gathered exclusively from this study:https://pubmed.ncbi.nlm.nih.gov/2943232/
So, when I checked my 3a-Diol-G levels again a few months back, they were almost in the high-end levels, and my hair quality was worse. Probably because of the higher androgen activity on the scalp? (Could it be)
(August 2019: Started Topical Finasteride:Baseline levels of DHT: 541 pg/ml
Otober 2019: DHT levels:302 pg/ml
January 2020: DHT levels:298 pg/ml-After starting Oral Finasteride as well ( I was already on it for about 3 weeks-0.5mg 3 times/week)
May 2020: DHT levels:313 pg/ml-I jumped into 1mg daily from 31st of March. I jumped to 0.5mg everyday from mid February
June 2020: DHT levels:283 pg/ml- I even tested 3a-Diol-G which is far a strongest indicator than DHT alone. 3a-Diol-G is a major DHT metabolite and it can converts back to DHT as well. It came low at 1.3 ng/ml while the range is: 3.4-22.
September 2020: DHT levels: 383 pg/ml
January 2021: DHT levels: 550 pg/ml !!! Back to baseline!-I checked as well my PSA levels and it was 0.23 ng/ml but I don't have baseline levels unfortunately.
May 2021 (Latest): I skipped DHT this time and tested only 3a-Diol-G. I got a result of: 16.1 this time!!! It makes sense, doesn't it? Higher DHT->Higher metabolite->Higher Androgenicity overall. I assume that my DHT is still high!-----Upregulated 5AR+Upregulated potent Androgen metabolites (3a-diol-G), destructive combination I guess.)
Another study (males this time):
"Serum 3a-androstanediol glucuronide (3a-Adiol-G) is considered to be an indicator of peripheral tissue androgen metabolism. Precursor circulating androgens are converted in peripheral tissue to dihydrotestosterone (DHT), which is ultimately metabolized to 3a-Adiol-G and secreted from the cell. Elevated serum 3a-Adiol-G concentrations have been reported in women in hyperandrogenic states. We studied 44 consecutive male medical students for chest hair density, acne, and serum dehydroepiandrosterone sulfate (DHEA-S), total testosterone (total T), free and albumin-bound (bioavailable) T (bio T), and 3a-Adiol-G concentrations. Although there was a considerable overlap of serum 3a-Adiol-G values among the groups defined by hair density or acne scores, we found statistically significant correlations between serum 3a-Adiol-G and chest hairiness (P = 0.0034), acne (P = 0.0005), and a combined chest hairiness and acne score (P = 0.0018). There was no significant correlation between these clinical parameters and the levels of precursor androgens. There was, however, a strong correlation between serum 3a-Adiol-G and bio T (P = 0.0005), suggesting that in men serum 3a-Adiol-G levels may be dependent upon available free and albumin-bound T. The correlations in men of serum 3a-Adiol-G with chest hair density, acne, and the hairiness and acne index supports the hypothesis that the serum levels of 3a-Adiol-G reflect the extent of androgen action in peripheral tissues."
Study:https://pubmed.ncbi.nlm.nih.gov/2972739/
As for the estrogen receptor alpha, I can't provide much information because it is somewhat complex. I will provide though one of the most detailed studies about Estrogens on hair follicles: https://academic.oup.com/edrv/article/27/6/677/2355194 (I will stand that the effects seem to be HIGHLY gender-sex specific and different when it comes to animals and humans. At least this is my understanding. Also, maybe Alfatradiol potency is not great for the ERα effects, since it is not as strong as 17b-Estradiol. Like @pariesienne said above, 17b inhibited hair growth while 17a not. and this is mentioned in the above study as well: Oh and Smart found that, in mice, topical E2 administration to clipped dorsal skin arrested hair follicles in telogen and produced a profound and prolonged inhibition of hair growth, whereas treatment with the biologically inactive stereoisomer 17α-estradiol did not alter hair growth.)
I will include as well a translated german study for Alfatradiol. Is one of the most detailed because there are males as well.
*Link for the 2005 study: https://www.thieme-connect.de/products/ejournals/html/10.1055/s-2005-870188
*Use a pdf translator.
TL;DR: Aromatase is very important for hair follicles, as we know. Estrogen Receptors Alpha and Beta are still kind of complex for me to explain them better and more detailed. 3a-Diol-G is probably a great indicator for androgen activity in tissues, including probably the scalp as well. Maybe it's worth it for someone to give at least a try to Alfatradiol, as it should stop testosterone and androstenedione as well to some extent, but not to Alfatradiol alone. Combination therapy is always the point. You have to connect as many dots as you can with all the mechanisms available.
Thanks for reading and sorry for any mistakes.
Last edited: