Thanks Hellouser to post it here

I can't make a thread as I'm a new member.
But I can post a new thing that i Found and I don't share on BTT, their abstract:
K. Washenik and Cotsarelis Abstract:
Prostaglandin D[SUB]2[/SUB] (PGD[SUB]2[/SUB]) and its synthesizing enzyme, PGD[SUB]2[/SUB] synthase, are present at higher levels in balding versus non-balding scalp in men with androgenetic alopecia. Our previous observations (Garza, 2012) in a mouse model that PGD[SUB]2[/SUB] inhibits hair growth via CRTH2/ PTGDR2, one of two PGD[SUB]2[/SUB] receptors, led us to hypothesize that PTGDR2 is the key receptor mediating the hair growth inhibitory activity of PGD[SUB]2[/SUB] in human follicles. In this study we tested several pharmacological PTGDR2 antagonists for their anti-PGD[SUB]2[/SUB] activity on human hair growth
in vitro. We found that PTGDR2 antagonists reversed the growth inhibition mediated by PGD[SUB]2[/SUB], in a dose-dependent manner (p<0.01), by reducing PGD[SUB]2[/SUB]-triggered apoptosis and maintaining keratinocyte proliferation. Topical administration of PGD[SUB]2[/SUB] to mice resulted in shortening of the anagen phase and accelerated entry into catagen, while applying a PTGDR2 antagonist to mice extended anagen phase, resulting in longer hair. RNA-Seq analysis on cultured human hair follicles showed decreased expression of hair follicle progenitor cell markers, such as CD34 and K19, in the PGD[SUB]2[/SUB] treated group. FACS analysis of mouse skin cells showed decreased Ki67-positive cells in the secondary hair germ population prior to anagen re-entry in PGD[SUB]2[/SUB] treated mice (p=0.029).
These results suggest that PGD[SUB]2[/SUB] suppresses the activation of the secondary germ/ hair progenitor cells. Our findings further underscore the key role of PGD[SUB]2[/SUB] in regulating hair growth and indicate that pharmacological antagonism of
PTGDR2 may be an effective approach in preventing and/or treating alopecia in patients sensitive to PGD[SUB]2[/SUB].
Interesting. CRTH2, when bound to PGD2, can promote the nuclear translocation of NFATc1.
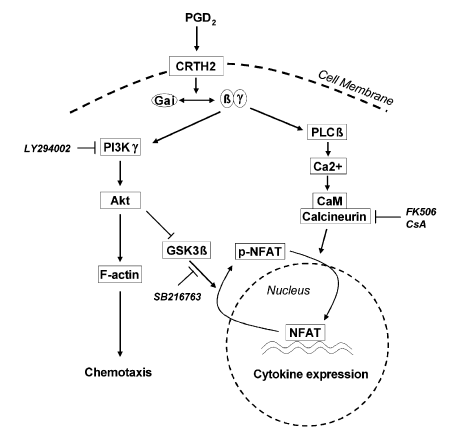
Fig. 8 – Scheme summarizing the proposed signal
pathways used by PGD2/CRTH2 to activate human Th2
cells. Activation of CRTH2 by PGD2 causes separation of
GTP-bound Gai protein from the receptor and Gbg
subunits. Consequently Gbg subunits stimulate effector
molecules, which include PI3Kg and PLCb. Activation of
PI3Kg leads to phosphorylation of Akt and re-organization
of cell skeleton including actin polymerization that is
required by chemotaxis. Inhibition of the PI3K pathway by
LY294002 attenuates downstream actin polymerization
and chemotaxis. Gbg-stimulated PLCb generates IP3,
which elicit Ca2+ influx into the cytosol. Liberated Ca2+
binds CaM that activates phosphatase calcineurin, which
in turn dephosphorylates NFAT inducing its activation
and translocation to the nucleus, and resulting in cytokine
gene transcription. Inhibition of calcineurin with FK506 or
CsA blocks NFAT nuclear translocation and cytokine
production. PI3K signals are also involved in the
regulation of NFAT via GSK-3b. Activated Akt
phosphorylates and inactivates GSK-3b. Inhibition of GSK-
3b phosphorylation promotes NFAT re-phosphorylation
and nuclear export. Blockade of GSK-3b activity with
SB216763 prolongs the duration of NFAT nuclear residence
and enhances gene transcription.
Source for the above:
Inhibition of PI3K and calcineurin suppresses chemoattractant receptor-homologous molecule expressed on Th2 cells (CRTH2)-dependent responses of Th2 lymphocytes to prostaglandin D(2)
And...
NFATc1 balances quiescence and proliferation of skin stem cells
Quiescent adult stem cells reside in specialized niches where they become activated to proliferate and differentiate during tissue homeostasis and injury. How stem cell quiescence is governed is poorly understood. We report here that NFATc1 is preferentially expressed by hair follicle stem cells in their niche, where its expression is activated by BMP signaling upstream and it acts downstream to transcriptionally repress CDK4 and maintain stem cell quiescence. As stem cells become activated during hair growth, NFATc1 is downregulated, relieving CDK4 repression and activating proliferation. When calcineurin/NFATc1 signaling is suppressed, pharmacologically or via complete or conditional NFATc1 gene ablation, stem cells are activated prematurely, resulting in precocious follicular growth. Our findings may explain why patients receiving cyclosporine A for immunosuppressive therapy display excessive hair growth, and unveil a functional role for calcium-NFATc1-CDK4 circuitry in governing stem cell quiescence.
Activation of β-catenin/TCF/Lef1 target genes is required for bulge stem cell activation and maintenance...
Here's another paper showing that nuclear NFAT can bind to Dishevelled (Dvl) and prevent the beta-catenin complex from transcribing its target genes:
http://www.jbc.org/content/286/43/37399.full
The Ca2+ signaling pathway appears to regulate the processes of the early development through its antagonism of canonical Wnt/β-catenin signaling pathway. However, the underlying mechanism is still poorly understood. Here, we show that nuclear factor of activated T cells (NFAT), a component of Ca2+ signaling, interacts directly with Dishevelled (Dvl) in a Ca2+-dependent manner. A dominant negative form of NFAT rescued the inhibition of the Wnt/β-catenin pathway triggered by the Ca2+ signal. NFAT functioned downstream of β-catenin without interfering with its stability, but influencing the interaction of β-catenin with Dvl by its competitively binding to Dvl. Furthermore, we demonstrate that NFAT is a regulator in the proliferation and differentiation of neural progenitor cells by modulating canonical Wnt/β-catenin signaling pathway in the neural tube of chick embryo. Our findings suggest that NFAT negatively regulates canonical Wnt/β-catenin signaling by binding to Dvl, thereby participating in vertebrate neurogenesis.
Previously, it has been reported that Ca2+ signaling or Wnt/Ca2+ signaling can block the canonical Wnt signaling transduction through an activation of NFAT in Xenopus embryos (23). However, the underlying mechanism is not clear. Here, we report that NFAT participates in the inhibition of Wnt signaling through direct interaction with Dvl in the nucleus. The binding of NFAT to Dvl prevents Dvl recruiting to the β-catenin transcription complex, thus reducing the transcriptional activity of β-catenin. Furthermore, our data also reveal that the cross-talk between NFAT and Wnt signaling is involved in the proliferation and differentiation of neural progenitor cells in the neural tube of chick embryo.
So perhaps this is the mechanism by which PGD2 inhibits bulge cell activation. PGD2 binds to CRTH2, which promotes the nuclear translocation of NFAT, preventing bulge cell activation (possibly by binding to Dvl and preventing transcription of Wnt target genes)?
- - - Updated - - -
By the way, that second paper shows that NFAT1c can bind to the CDK4 promoter and repress its transcription. This would stop the cell cycle progression from G1 to S independent of any effect on beta-catenin.