squeegee
Banned
- Reaction score
- 132
Endocrinology. 1996 Jan;137(1):105-12.
Induction of lipid peroxidation during steroidogenesis in the rat testis.
Peltola V, Huhtaniemi I, Metsa-Ketela T, Ahotupa M.
Source
Department of Physiology, University of Turku, Finland.
Abstract
Free radical production and lipid peroxidation are potentially important mediators in testicular physiology and toxicology. The cytochrome P450 enzymes of the steroidogenic pathway are known to produce free radicals. The present study was conducted to elucidate in vivo the gonadotropin regulation of free radical-mediated lipid peroxidation and the antioxidative defense system in the rat testis. GnRH antagonist (Org 30276; 1 mg/kg BW) and testosterone [40-mm SILASTIC brand (Dow-Corning) capsules] treatments were used to suppress serum gonadotropin levels. As expected, serum LH decreased to a very low level, whereas serum FSH decreased only slightly. Testosterone treatment for 8 days decreased the levels of the peroxide-metabolizing enzymes, catalase, glutathione peroxidase (GSH-Px), and glutathione transferase (-44%, -24%, and -31%, respectively; P < 0.01 for all). These changes predominately reflect the interstitial tissue, in which catalase and GSH-Px activities were much higher than in the seminiferous tubules. Testicular CuZn or Mn superoxide dismutase activities, which were high in the seminiferous tubules, were not affected by gonadotropin suppression. The total peroxyl radical-trapping capacity of the testis, or its components, vitamin E and ubiquinol 9, were not affected either. Lipid peroxidation was decreased after 8-day treatment, as detected by diminished formation of conjugated dienes and fluorescent chromolipids (-30% and -19%, respectively; P < 0.05 for both). Similar results of decreasing catalase and GSH-Px activities were found after gonadotropin suppression with GnRH antagonist treatment for 2 days or testosterone treatment for 5 days. Substitution with hCG, alone or in combination with recombinant human FSH, reversed the changes in enzyme activities, whereas FSH alone had no effect. After 5-day testosterone treatment, catalase messenger RNA expression was studied by Northern hybridization, and it was observed to parallel the changes in enzyme activity. The site of free radical production was studied by separating interstitial tissue and seminiferous tubules 5 h after hCG injection. GSH-Px was induced by hCG only in the interstitial tissue (+28%; P< 0.01), supporting the hypothesis of free radical production during steroidogenesis. Aminoglutethimide, an inhibitor of the P450 cholesterol side-chain cleavage enzyme, induced extensive lipid peroxidation in the testis. Presumably, aminoglutethimide leads to leakage of free radicals from the P450 enzyme when substrate oxygenation is prevented. In conclusion, the present .
Steroidogenic isoenzymes in human hair and their potential role in androgenetic alopecia.
Abstract
Androgenetic alopecia (Androgenetic Alopecia) is the most common type of hair loss. The relatively strong concordance of the degree of baldness in fathers and sons is not consistent with a simple Mendelian trait, and a polygenic basis is considered to be most likely. So far, the predisposing genes for Androgenetic Alopecia are unknown and we do not understand the molecular steps involved in androgen-dependent beard growth versus androgen-dependent hair loss, but Androgenetic Alopecia can be defined as a dihydrotestosterone (DHT)-dependent process with continuous miniaturization of sensitive hair follicles. The type 2 5alpha-reductase plays a central role by the intrafollicular conversion of testosterone to DHT. However, due to the increasing knowledge in this field, we now know that there are many more steroidogenic enzymes involved in the onset and development of Androgenetic Alopecia, and this article shall provide a critical overview of recent discoveries.
Copyright 2003 S. Karger AG, Basel
Higher Levels of Steroidogenic Acute Regulatory Protein and Type I 3
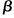 -Hydroxysteroid Dehydrogenase in the Scalp of Men with Androgenetic Alopecia
-Hydroxysteroid Dehydrogenase in the Scalp of Men with Androgenetic Alopecia
http://www.nature.com/jid/journal/v126/n10/full/5700442a.html
Steroidogenic enzymes in skin.
S Andersson
Department of Obstetrics-Gynecology and Biochemistry, University of Texas Southwestern Medical Center, 5323 Harry Hines Boulevard, Dallas, TX 75390-9032, USA.
The gonadal synthesis of testosterone from cholesterol involves four enzymes, namely, cytochrome P-450 side-chain cleavage enzyme, cytochrome P-450 17a-hydroxylase/lyase, 3b-hydroxysteroid dehydrogenase, and 17b-hydroxysteroid dehydrogenase. A significant part of the plasma-borne testosterone is converted in androgen target tissues, such as the skin, to the more potent androgen dihydrotestosterone by the steroid 5a-reductase type 1 and type 2 isoenzymes. Dihydrotestosterone, which binds to the nuclear androgen receptor with much greater affinity than testosterone, is the androgen responsible for a process leading to androgenetic alopecia. Consequently, the 5a-reductase inhibitor finasteride was developed and has proven efficacious in promoting hair growth as a consequence of lowering scalp and plasma dihydrotestosterone levels. In contrast to the direct synthesis of dihydrotestosterone from testosterone, biologically inactive C19-steroids produced by glandular and peripheral tissues may also feed into the scalp skin production of dihydrotestosterone by the local expression of reductive 17b-hydroxysteroid dehydrogenase, oxidative 3a-hydroxysteroid dehydrogenase, and 3b-hydroxysteroid dehydrogenase enzymes. Aberrant expression of one or more of these enzymes, could conceivably result in increased scalp dihydrotestosterone levels, and possibly, acceleration of the balding process in genetically predisposed men and women.
Induction of lipid peroxidation during steroidogenesis in the rat testis.
Peltola V, Huhtaniemi I, Metsa-Ketela T, Ahotupa M.
Source
Department of Physiology, University of Turku, Finland.
Abstract
Free radical production and lipid peroxidation are potentially important mediators in testicular physiology and toxicology. The cytochrome P450 enzymes of the steroidogenic pathway are known to produce free radicals. The present study was conducted to elucidate in vivo the gonadotropin regulation of free radical-mediated lipid peroxidation and the antioxidative defense system in the rat testis. GnRH antagonist (Org 30276; 1 mg/kg BW) and testosterone [40-mm SILASTIC brand (Dow-Corning) capsules] treatments were used to suppress serum gonadotropin levels. As expected, serum LH decreased to a very low level, whereas serum FSH decreased only slightly. Testosterone treatment for 8 days decreased the levels of the peroxide-metabolizing enzymes, catalase, glutathione peroxidase (GSH-Px), and glutathione transferase (-44%, -24%, and -31%, respectively; P < 0.01 for all). These changes predominately reflect the interstitial tissue, in which catalase and GSH-Px activities were much higher than in the seminiferous tubules. Testicular CuZn or Mn superoxide dismutase activities, which were high in the seminiferous tubules, were not affected by gonadotropin suppression. The total peroxyl radical-trapping capacity of the testis, or its components, vitamin E and ubiquinol 9, were not affected either. Lipid peroxidation was decreased after 8-day treatment, as detected by diminished formation of conjugated dienes and fluorescent chromolipids (-30% and -19%, respectively; P < 0.05 for both). Similar results of decreasing catalase and GSH-Px activities were found after gonadotropin suppression with GnRH antagonist treatment for 2 days or testosterone treatment for 5 days. Substitution with hCG, alone or in combination with recombinant human FSH, reversed the changes in enzyme activities, whereas FSH alone had no effect. After 5-day testosterone treatment, catalase messenger RNA expression was studied by Northern hybridization, and it was observed to parallel the changes in enzyme activity. The site of free radical production was studied by separating interstitial tissue and seminiferous tubules 5 h after hCG injection. GSH-Px was induced by hCG only in the interstitial tissue (+28%; P< 0.01), supporting the hypothesis of free radical production during steroidogenesis. Aminoglutethimide, an inhibitor of the P450 cholesterol side-chain cleavage enzyme, induced extensive lipid peroxidation in the testis. Presumably, aminoglutethimide leads to leakage of free radicals from the P450 enzyme when substrate oxygenation is prevented. In conclusion, the present .
Steroidogenic isoenzymes in human hair and their potential role in androgenetic alopecia.
Abstract
Androgenetic alopecia (Androgenetic Alopecia) is the most common type of hair loss. The relatively strong concordance of the degree of baldness in fathers and sons is not consistent with a simple Mendelian trait, and a polygenic basis is considered to be most likely. So far, the predisposing genes for Androgenetic Alopecia are unknown and we do not understand the molecular steps involved in androgen-dependent beard growth versus androgen-dependent hair loss, but Androgenetic Alopecia can be defined as a dihydrotestosterone (DHT)-dependent process with continuous miniaturization of sensitive hair follicles. The type 2 5alpha-reductase plays a central role by the intrafollicular conversion of testosterone to DHT. However, due to the increasing knowledge in this field, we now know that there are many more steroidogenic enzymes involved in the onset and development of Androgenetic Alopecia, and this article shall provide a critical overview of recent discoveries.
Copyright 2003 S. Karger AG, Basel
Higher Levels of Steroidogenic Acute Regulatory Protein and Type I 3
http://www.nature.com/jid/journal/v126/n10/full/5700442a.html
Steroidogenic enzymes in skin.
S Andersson
Department of Obstetrics-Gynecology and Biochemistry, University of Texas Southwestern Medical Center, 5323 Harry Hines Boulevard, Dallas, TX 75390-9032, USA.
The gonadal synthesis of testosterone from cholesterol involves four enzymes, namely, cytochrome P-450 side-chain cleavage enzyme, cytochrome P-450 17a-hydroxylase/lyase, 3b-hydroxysteroid dehydrogenase, and 17b-hydroxysteroid dehydrogenase. A significant part of the plasma-borne testosterone is converted in androgen target tissues, such as the skin, to the more potent androgen dihydrotestosterone by the steroid 5a-reductase type 1 and type 2 isoenzymes. Dihydrotestosterone, which binds to the nuclear androgen receptor with much greater affinity than testosterone, is the androgen responsible for a process leading to androgenetic alopecia. Consequently, the 5a-reductase inhibitor finasteride was developed and has proven efficacious in promoting hair growth as a consequence of lowering scalp and plasma dihydrotestosterone levels. In contrast to the direct synthesis of dihydrotestosterone from testosterone, biologically inactive C19-steroids produced by glandular and peripheral tissues may also feed into the scalp skin production of dihydrotestosterone by the local expression of reductive 17b-hydroxysteroid dehydrogenase, oxidative 3a-hydroxysteroid dehydrogenase, and 3b-hydroxysteroid dehydrogenase enzymes. Aberrant expression of one or more of these enzymes, could conceivably result in increased scalp dihydrotestosterone levels, and possibly, acceleration of the balding process in genetically predisposed men and women.
