- Reaction score
- 984
I don’t know. It is positive that the CEO is experienced and that they Want to they in Great britain. But negative that No Big company/Investor like Shiseido is in. Let’s Hope they will successWhat's your opinion on Hairclone?
I don’t know. It is positive that the CEO is experienced and that they Want to they in Great britain. But negative that No Big company/Investor like Shiseido is in. Let’s Hope they will successWhat's your opinion on Hairclone?
If it is the goal, it is easy, prevention better than cure, and JL & JO know about it, Grow your Hairi feel with the community. for me maintenance also wouldnt be enoughthats a shame... but people have to realize that Shiseido found the cure for all upcoming generations...
Bla bla bla, useless and annoying rantI am trying to improve the quality of posters on the forum. There's a reason the group buy guys left for the discord and a large part is because the average poster here contributes nothing, and only asks sh*t like "where/what is x?", which has been answered countless times on this very forum and which can easily be googled.
Internet forums work best as give and take. If you refuse to do basic research you are a literal leech.
If it is the goal, it is easy, prevention better than cure, and JL & JO know about it, Grow your Hair
Good luck with that stock. This is a crappy company with a train wreck of a balance sheet. If they don't file for bankruptcy soon ill be shocked.
RepliCel Files Notice of Arbitration against Shiseido Company to Resolve its License Agreement Dispute Regarding its Androgenetic Alopecia Cell Therapy
After several unsuccessful attempts to settle the dispute, RepliCel has filed an arbitration claim seeking Shiseido's full compliance with the agreement or return of the license and all collaboration data and innovations related to its cell therapy treatment for male and female pattern hair...www.accesswire.com
RepliCel Files Notice of Arbitration against Shiseido Company to Resolve its License Agreement Dispute Regarding its Androgenetic Alopecia Cell Therapy
It is obvious that Shiseido breached the contract because they had to share the Phase 2 data with RepliCel But Shiseido didnt.
So Replicel will win the Court against Shiseido. After that it will be very interesting because then Shiseido has to share their data with Replicel. The other Option is that Shiseido will Buy the global rights of RCH-01.
Shiseido is doing a Phase 3 dose-finding trial. In the Phase II trial it was noticed that lower Cell concentrations of dermal sheath cup cells will lead to the best results.
Shiseido is now trialing with multiple injections and lower dosis. It could reach much better results.
If Shiseido is satisfied with the recently ongoing Phase 3 trial with lower doses and multiple injections then they wouldnt share the data with Replicel. They would buy the global rights of replicel.
So i just bought some RepliCel Shares at this Low price- Let’s See what will Happen(0.3$ —> 20$ )


There was no placebo group to compare their results to so there's no guarantee this is going to be able to maintain our hair but let's hope it's still at least better than tap water. So where do you see this leading? Do you think this is something we'll see on the market in the near future?FINAL SHISEIDO PHASE III STUDY RELEASE:

Efficacy of autologous dermal sheath cup cell transplantation in male and female pattern hair loss: A Single-Arm, Multi-Center, phase III equivalent clinical study - PubMed
A previous, proof-of-concept clinical study suggested that dermal sheath cup cell injections into the affected areas of male/female pattern hair loss (PHL) may have some amelioratory effects, the clinical efficacy of which needs further examination. A phase III equivalent clinical study was...pubmed.ncbi.nlm.nih.gov
It's garbage.There was no placebo group to compare their results to so there's no guarantee this is going to be able to maintain our hair but let's hope it's still at least better than tap water. So where do you see this leading? Do you think this is something we'll see on the market in the near future?
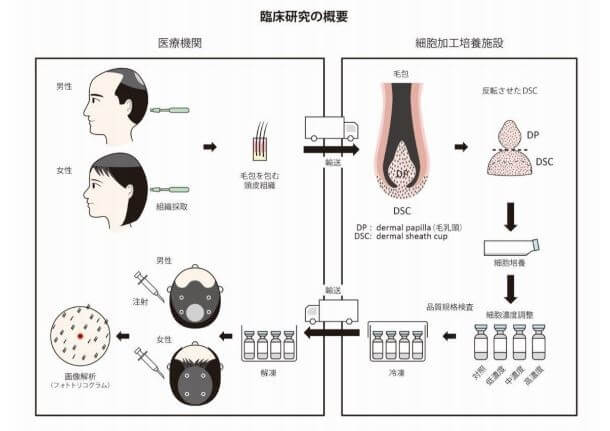
How many patients got worse though or at least maintained? If it maintains hair that would still be nice but again the sample size was so small and they don't even compare it to a placebo. These results are mostly meaningless, guess I'm stuck on RU and top fina until HMI 115 is releasedIt's garbage.

Shiseido Phase 3 Trial Results | Hair Loss Cure 2020
Shiseido (Japan) has published the results of its Phase III equivalent study on the efficacy of autologous dermal sheath cup cell transplantation for hair loss.www.hairlosscure2020.com
On global photographic assessment, 30% of the participants showed improvement.
The phototricogram data analysis showed increases in the:
- Cumulative hair diameter of 107.6 ± 152.6 μm/cm2. This was a +1.4% increase versus baseline.
- Hair cross-sectional area of 3069.1 ± 10960.7 μm2 /cm2. This was a +3.4% increase versus baseline.
- Mean hair diameter of 0.9 ± 0.9 μm. This was a +2.2% increase versus baseline.
