http://www.histogeninc.com/products/hgen_001.htm
HGEN-001 HGEN-001 is a proprietary liquid formula created by infusing the soluble chemicals secreted by the newborn fibroblasts with the liquid media. It is the first naturally stabilized and bioactive formulation of wnt proteins and growth factors, offering exceptional opportunities for applications including research tools and biologics products.
[*]...The formula contains naturally stabilized and bioactive wnt proteins and activity
[*]...The formula contains naturally secreted growth factors
[*]...HGEN-001 is soluble and injectable
[*]...The formula requires no purification process
[*]...Capabilities for Hair growth
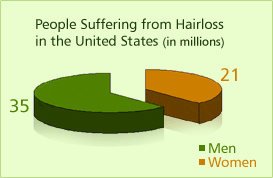
Hair loss affects 35 million men and 21 million women in the United States.
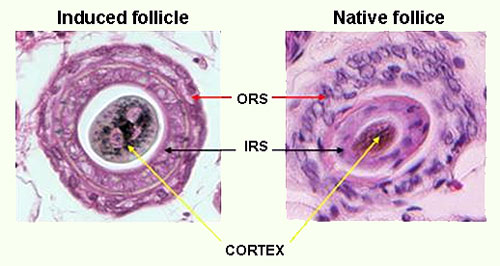
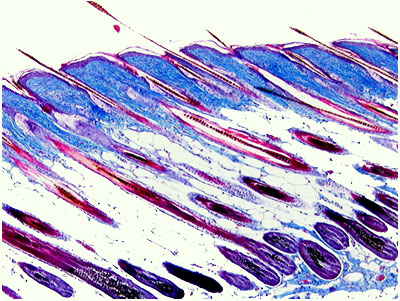
One of Histogen's significant applications for HGEN-001 is its utilization as an injectable for hair growth. The combination of wnt proteins and growth factors making up HGEN-001 has been shown to both stimulate resting hair follicles and induce new follicle formation.
In May 2007, these findings were substantiated by research performed by Dr. George Cotsarelis, which offered further evidence that these growth factors and proteins in combination lead to a significant increase in the creation of new hair follicles in mice.
Histogen's injectable for hair growth will be undergoing clinical trials, and launch is planned for 2015.
HGEN-001 HGEN-001 is a proprietary liquid formula created by infusing the soluble chemicals secreted by the newborn fibroblasts with the liquid media. It is the first naturally stabilized and bioactive formulation of wnt proteins and growth factors, offering exceptional opportunities for applications including research tools and biologics products.
[*]...The formula contains naturally stabilized and bioactive wnt proteins and activity
[*]...The formula contains naturally secreted growth factors
[*]...HGEN-001 is soluble and injectable
[*]...The formula requires no purification process
[*]...Capabilities for Hair growth
Hair loss affects 35 million men and 21 million women in the United States.
One of Histogen's significant applications for HGEN-001 is its utilization as an injectable for hair growth. The combination of wnt proteins and growth factors making up HGEN-001 has been shown to both stimulate resting hair follicles and induce new follicle formation.
In May 2007, these findings were substantiated by research performed by Dr. George Cotsarelis, which offered further evidence that these growth factors and proteins in combination lead to a significant increase in the creation of new hair follicles in mice.
Histogen's injectable for hair growth will be undergoing clinical trials, and launch is planned for 2015.